Now published, see post #37
----------------------------------------------
Low Dose Rapamycin Alleviates Clinical Symptoms of Fatigue and PEM in ME/CFS Patients via Improvement of Autophagy
Brian T. Ruan, Sarojini Bulbule, Amy Reyes, Bela Chheda, Lucinda Bateman, Jennifer Bell, Braydon Yellman, Stephanie Grach, Jon Berner, Daniel L. Peterson, David Kaufman, Avik Roy, C. Gunnar Gottschalk
Background
mTOR activation is associated with chronic inflammation in ME/CFS. Previous studies have shown that sustained mTOR activation can cause chronic muscle fatigue by inhibiting ATG13-mediated autophagy. This highlights the pivotal role of mTOR in the pathogenesis of ME/CFS.
Methods
We conducted a decentralized, uncontrolled trial of rapamycin in 86 patients with ME/CFS to evaluate its safety and efficacy. Low-dose rapamycin (6 mg/kg) was administered, and core ME/CFS symptoms were assessed on days 30 (T1), 60 (T2), and 90 (T3). Plasma levels of autophagy metabolites, such as pSer258-ATG13 and BECLIN-1, were measured and correlated with clinical outcomes, specifically MFI.
Results
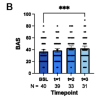
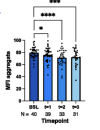
Rapamycin (6 mg/week) was tolerated without any SAEs. Of the 40 patients, 29 (72.5%) showed strong recovery in PEM, fatigue, and OI, along with improvements in MFI fatigue domains and SF-36 aspects. High levels of BECLIN-1 were detected in T3. Plasma pSer258-ATG13 levels were strongly downregulated at T1. Spearman’s correlation analysis indicated an association between autophagy impairment and reduced activity.
Conclusions
Low-dose rapamycin effectively reduced PEM and other key symptoms in patients with ME/CFS, as measured by BAS, SSS, MFI, and SF-36. Future studies should encompass dose optimization and develop a diagnostic tool to identify responders with mTOR-mediated autophagy disruption.
Link | PDF (Preprint: ResearchSquare) [Open Access]
----------------------------------------------
Low Dose Rapamycin Alleviates Clinical Symptoms of Fatigue and PEM in ME/CFS Patients via Improvement of Autophagy
Brian T. Ruan, Sarojini Bulbule, Amy Reyes, Bela Chheda, Lucinda Bateman, Jennifer Bell, Braydon Yellman, Stephanie Grach, Jon Berner, Daniel L. Peterson, David Kaufman, Avik Roy, C. Gunnar Gottschalk
Background
mTOR activation is associated with chronic inflammation in ME/CFS. Previous studies have shown that sustained mTOR activation can cause chronic muscle fatigue by inhibiting ATG13-mediated autophagy. This highlights the pivotal role of mTOR in the pathogenesis of ME/CFS.
Methods
We conducted a decentralized, uncontrolled trial of rapamycin in 86 patients with ME/CFS to evaluate its safety and efficacy. Low-dose rapamycin (6 mg/kg) was administered, and core ME/CFS symptoms were assessed on days 30 (T1), 60 (T2), and 90 (T3). Plasma levels of autophagy metabolites, such as pSer258-ATG13 and BECLIN-1, were measured and correlated with clinical outcomes, specifically MFI.
Results
Rapamycin (6 mg/week) was tolerated without any SAEs. Of the 40 patients, 29 (72.5%) showed strong recovery in PEM, fatigue, and OI, along with improvements in MFI fatigue domains and SF-36 aspects. High levels of BECLIN-1 were detected in T3. Plasma pSer258-ATG13 levels were strongly downregulated at T1. Spearman’s correlation analysis indicated an association between autophagy impairment and reduced activity.
Conclusions
Low-dose rapamycin effectively reduced PEM and other key symptoms in patients with ME/CFS, as measured by BAS, SSS, MFI, and SF-36. Future studies should encompass dose optimization and develop a diagnostic tool to identify responders with mTOR-mediated autophagy disruption.
Link | PDF (Preprint: ResearchSquare) [Open Access]
Last edited: