SARS-CoV-2 spike protein receptor-binding domain perturbates intracellular calcium homeostasis and impairs pulmonary vascular endothelial cells
Yang, Kai; Liu, Shiyun; Yan, Han; Lu, Wenju; Shan, Xiaoqian; Chen, Haixia; Bao, Changlei; Feng, Huazhuo; Liao, Jing; Liang, Shuxin; Xu, Lei; Tang, Haiyang; Yuan, Jason X.-J.; Zhong, Nanshan; Wang, Jian
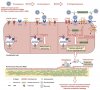
Exposure to the spike protein or receptor-binding domain (S-RBD) of SARS-CoV-2 significantly influences endothelial cells and induces pulmonary vascular endotheliopathy. In this study, angiotensin-converting enzyme 2 humanized inbred (hACE2 Tg) mice and cultured pulmonary vascular endothelial cells were used to investigate how spike protein/S-RBD impacts pulmonary vascular endothelium.
Results show that S-RBD leads to acute-to-prolonged induction of the intracellular free calcium concentration ([Ca2+]i) via acute activation of TRPV4, and prolonged upregulation of mechanosensitive channel Piezo1 and store-operated calcium channel (SOCC) key component Orai1 in cultured human pulmonary arterial endothelial cells (PAECs). In mechanism, S-RBD interacts with ACE2 to induce formation of clusters involving Orai1, Piezo1 and TRPC1, facilitate the channel activation of Piezo1 and SOCC, and lead to elevated apoptosis. These effects are blocked by Kobophenol A, which inhibits the binding between S-RBD and ACE2, or intracellular calcium chelator, BAPTA-AM. Blockade of Piezo1 and SOCC by GsMTx4 effectively protects the S-RBD-induced pulmonary microvascular endothelial damage in hACE2 Tg mice via normalizing the elevated [Ca2+]i. Comparing to prototypic strain, Omicron variants (BA.5.2 and XBB) of S-RBD induces significantly less severe cell apoptosis. Transcriptomic analysis indicates that prototypic S-RBD confers more severe acute impacts than Delta or Lambda S-RBD.
In summary, this study provides compelling evidence that S-RBD could induce persistent pulmonary vascular endothelial damage by binding to ACE2 and triggering [Ca2+]i through upregulation of Piezo1 and Orai1. Targeted inhibition of ACE2-Piezo1/SOCC-[Ca2+]i axis proves a powerful strategy to treat S-RBD-induced pulmonary vascular diseases.
Link | PDF (Nature Signal Transduction and Targeted Therapy)
Yang, Kai; Liu, Shiyun; Yan, Han; Lu, Wenju; Shan, Xiaoqian; Chen, Haixia; Bao, Changlei; Feng, Huazhuo; Liao, Jing; Liang, Shuxin; Xu, Lei; Tang, Haiyang; Yuan, Jason X.-J.; Zhong, Nanshan; Wang, Jian
Exposure to the spike protein or receptor-binding domain (S-RBD) of SARS-CoV-2 significantly influences endothelial cells and induces pulmonary vascular endotheliopathy. In this study, angiotensin-converting enzyme 2 humanized inbred (hACE2 Tg) mice and cultured pulmonary vascular endothelial cells were used to investigate how spike protein/S-RBD impacts pulmonary vascular endothelium.
Results show that S-RBD leads to acute-to-prolonged induction of the intracellular free calcium concentration ([Ca2+]i) via acute activation of TRPV4, and prolonged upregulation of mechanosensitive channel Piezo1 and store-operated calcium channel (SOCC) key component Orai1 in cultured human pulmonary arterial endothelial cells (PAECs). In mechanism, S-RBD interacts with ACE2 to induce formation of clusters involving Orai1, Piezo1 and TRPC1, facilitate the channel activation of Piezo1 and SOCC, and lead to elevated apoptosis. These effects are blocked by Kobophenol A, which inhibits the binding between S-RBD and ACE2, or intracellular calcium chelator, BAPTA-AM. Blockade of Piezo1 and SOCC by GsMTx4 effectively protects the S-RBD-induced pulmonary microvascular endothelial damage in hACE2 Tg mice via normalizing the elevated [Ca2+]i. Comparing to prototypic strain, Omicron variants (BA.5.2 and XBB) of S-RBD induces significantly less severe cell apoptosis. Transcriptomic analysis indicates that prototypic S-RBD confers more severe acute impacts than Delta or Lambda S-RBD.
In summary, this study provides compelling evidence that S-RBD could induce persistent pulmonary vascular endothelial damage by binding to ACE2 and triggering [Ca2+]i through upregulation of Piezo1 and Orai1. Targeted inhibition of ACE2-Piezo1/SOCC-[Ca2+]i axis proves a powerful strategy to treat S-RBD-induced pulmonary vascular diseases.
Link | PDF (Nature Signal Transduction and Targeted Therapy)