Chandelier
Senior Member (Voting Rights)
Efficacy of heat-treated Lacticaseibacillus paracasei PS23 for individuals with long coronavirus disease-19 syndrome: a double-blinded randomized control pilot study - Scientific Reports
Long coronavirus disease (long COVID) refers to symptoms that persist beyond the acute phase of SARS-CoV-2 infection. This study applied the World Health Organization (WHO) definition, which identifies post-COVID-19 condition as symptoms lasting at least two months and beginning around three...
Full title:
Efficacy of heat-treated Lacticaseibacillus paracasei PS23 for individuals with long coronavirus disease-19 syndrome: a double-blinded randomized control pilot study
Shu-I Wu, Chen-Ju Lin, Ya-Ju Lin, I-Chieh Lin, Lee-Ching Hwang & Wan-Lin Chen
Abstract
Long coronavirus disease (long COVID) refers to symptoms that persist beyond the acute phase of SARS-CoV-2 infection.This study applied the World Health Organization (WHO) definition, which identifies post-COVID-19 condition as symptoms lasting at least two months and beginning around three months after confirmed infection.
Given limited evidence on probiotic use for long COVID, we conducted a double-blind, randomized, placebo-controlled trial to evaluate the effects of heat-treated Lacticaseibacillus paracasei PS23 (HT-PS23) in individuals with confirmed COVID-19 and self-reported prolonged symptoms.
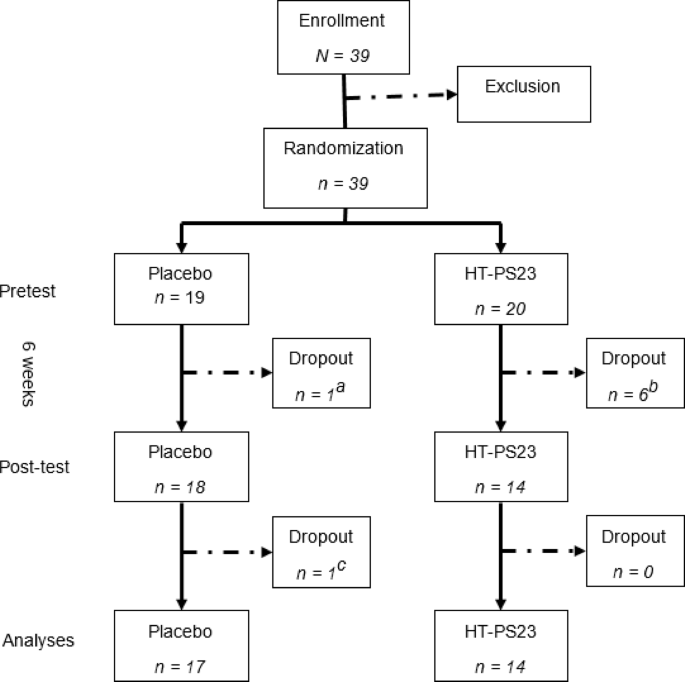
Thirty-nine eligible participants were randomly assigned to receive either the heat-treated Lacticaseibacillus paracaseiPS23 (HT-PS23) or placebo for six weeks.
Assessments included validated neuropsychological tests (including executive function and working memory using Color Trails 1&2 and Digit Span and Coding), self-reported symptom scales of depression, anxiety, and quality of life, and blood biomarkers related to inflammation, immune response, and stress (e.g. cortisol, IFN-γ zonulin).
After the six-week trial, HT-PS23 group showed notable improvements in cortisol level (p = 0.006), a reduction in errors of CTT2 (p = 0.018), and symptom severity related to breathing difficulties (p = 0.016) and appetite loss (p = 0.030) compared to the placebo group.
HT-PS23 may be a feasible and well-tolerated intervention for relieving cognitive or physical symptoms associated with long COVID.
Further studies with larger samples are warranted.
https://doi.org/10.1038/s41598-025-27036-3
