Bunker, T., Horne, B.D., Baldwin, M.D. et al. Intermittent fasting and a no-sugar diet for Long COVID symptoms: a randomized crossover trial. Sci Rep 15, 27563 (2025). https://doi.org/10.1038/s41598-025-07461-0
Abstract
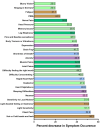
Long COVID (LC) is a common chronic health condition that impairs daily functioning and social connections. This is the first randomized clinical trial to directly compare the effect of two Intermittent Fasting regimens on LC symptoms. The main objectives of this 10-week randomized cross-over trial are to compare the efficacy and safety of 4 weeks of 1–2 day fasting plus a restricted diet vs 4 weeks of mild time-restricted eating (TRE) and a restricted diet in reducing patient-reported LC symptoms. After a 2-week run-in, subjects were randomized to treatment A (TRE) or treatment B (Fasting) for 4 weeks. Subjects then crossed over to the other treatment for 4 weeks. The median fasting duration was 38 h (night-day-night), and the mean duration was 42 h. Symptoms were assessed via weekly online surveys. Primary outcomes were changes in LC symptom severity scores (LC-Scores) and in the number of LC symptoms (numLCsym) between treatments. Secondary outcomes were changes in LC-Scores and numLCsym over the 10-week trial. Fasting was superior to TRE alone in reducing LC-Scores (p = 0.008). The numLCsym decreased − 5.0 during the Fasting 4 weeks vs − 1.4 in the TRE 4 weeks (p = 0.002). Altogether, the 10-week regimen of a no-sugar diet, TRE and Fasting decreased the mean LC-Score by 51.8% (p < 0.0001) from 37.8 to 18.2. Similarly, numLCsym decreased from 20.5 to 12.2, a decrease of 40.6% (p < 0.0001). No major adverse safety events were recorded. Both intermittent fasting interventions decreased symptoms over the 10-week trial but the more intense fasting regimen was significantly better.https://www.nature.com/articles/s41598-025-07461-0#Sec2