Dolphin
Senior Member (Voting Rights)
Free fulltext:
https://www.mdpi.com/2673-5601/2/3/33
Long COVID (PASC) Is Maintained by a Self-Sustaining Pro-Inflammatory TLR4/RAGE-Loop of S100A8/A9 > TLR4/RAGE Signalling, Inducing Chronic Expression of IL-1b, IL-6 and TNFa: Anti-Inflammatory Ezrin Peptides as Potential Therapy
by
Rupert Donald Holms
Newal R&D Ltd., London NW1 7SX, UK
Academic Editor: Stefano Aquaro
Immuno 2022, 2(3), 512-533; https://doi.org/10.3390/immuno2030033
Received: 1 August 2022 / Revised: 31 August 2022 / Accepted: 5 September 2022 / Published: 8 September 2022
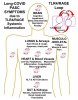
Abstract: Long COVID, also referred to as Post-Acute Sequelae of COVID (PASC), is probably triggered during SARS-CoV-2 infection and acute COVID-19 by SARS-CoV-2 Spike-protein binding and hyper-activating the cell-membrane expressed Receptor for Advance Glycation End-products (mRAGE) and Toll-Like Receptor 4 (TLR4). SARS-CoV-2 infects lung monocytes by Spike binding to mRAGE (not ACE2). During acute COVID-19, high levels of IL-6 hyper-stimulate S100A8/A9 expression and secretion. Although no viral protein nor mRNA can be detected in half of long COVID (PASC) patients, there is a significant elevation of serum levels of IL-1b, IL-6, TNFa, and S100A8/A9. It appears that a pathological pro-inflammatory feedback loop (the TLR4/RAGE-loop) is established during acute COVID-19, which is maintained by S100A8/A9 > RAGE/TLR4 chronic inflammatory signalling, even after SARS-CoV-2 has been cleared from the body. During long COVID/PASC, Ca2+-binding protein S100A8/A9 chronically stimulates TLR4/RAGE-signalling to induce chronic expression of IL-1b, IL-6 and TNFa. Secreted IL-6 binds to its IL-6R receptor on the surface of other cells and signals via STAT3 and C/EBPb for more S100A8/A9 expression. Secreted IL-1b binds to its receptor IL-1R on other cells, and signals via NFkB for more mRAGE and TLR4 expression. New S100A8/A9 can bind and activate cell-surface mRAGE and TLR4 to stimulate expression of more IL- 1b, IL-6 and TNFa. This process establishes a pathogenic pro-inflammatory TLR4/RAGE-loop: IL-1b + IL-6 > IL-1R + IL-6R > TLR4/mRAGE + S100A8/A9 > IL-1b + IL-6, which generates multi-organ inflammation that persists in the blood vessels, the brain, the liver, the heart, the kidneys, the gut and the musculo-skeletal system, and is responsible for all the complex pathologies associated with long COVID/PASC. Chronic expression of IL-1, IL-6 and TNFa is critical for the maintenance of the TLR4/RAGE-loop and persistence of long COVID/PASC. Ezrin peptides are inhibitors of IL-1, IL-6, IL-8 and TNFa expression, so are now being investigated as potential therapy for long COVID/PASC. There is preliminary anecdotal evidence of symptomatic relief (not confirmed yet by formal clinical trials) from a few long COVID/PASC patient volunteers, after treatment with ezrin peptide therapy. Keywords: long COVID; PASC; RAGE; TLR4; p38MAPK; IL-1b; IL-6; IL-8; TNFa; S100; S100A8/A9; AGE; HMGB1; ezrin peptide therapy; HEP-1; RepG3
Keywords: long COVID; PASC; RAGE; TLR4; p38MAPK; IL-1b; IL-6; IL-8; TNFa; S100; S100A8/A9; AGE; HMGB1; ezrin peptide therapy; HEP-1; RepG3
https://www.mdpi.com/2673-5601/2/3/33
Long COVID (PASC) Is Maintained by a Self-Sustaining Pro-Inflammatory TLR4/RAGE-Loop of S100A8/A9 > TLR4/RAGE Signalling, Inducing Chronic Expression of IL-1b, IL-6 and TNFa: Anti-Inflammatory Ezrin Peptides as Potential Therapy
by
Rupert Donald Holms
Newal R&D Ltd., London NW1 7SX, UK
Academic Editor: Stefano Aquaro
Immuno 2022, 2(3), 512-533; https://doi.org/10.3390/immuno2030033
Received: 1 August 2022 / Revised: 31 August 2022 / Accepted: 5 September 2022 / Published: 8 September 2022
Abstract: Long COVID, also referred to as Post-Acute Sequelae of COVID (PASC), is probably triggered during SARS-CoV-2 infection and acute COVID-19 by SARS-CoV-2 Spike-protein binding and hyper-activating the cell-membrane expressed Receptor for Advance Glycation End-products (mRAGE) and Toll-Like Receptor 4 (TLR4). SARS-CoV-2 infects lung monocytes by Spike binding to mRAGE (not ACE2). During acute COVID-19, high levels of IL-6 hyper-stimulate S100A8/A9 expression and secretion. Although no viral protein nor mRNA can be detected in half of long COVID (PASC) patients, there is a significant elevation of serum levels of IL-1b, IL-6, TNFa, and S100A8/A9. It appears that a pathological pro-inflammatory feedback loop (the TLR4/RAGE-loop) is established during acute COVID-19, which is maintained by S100A8/A9 > RAGE/TLR4 chronic inflammatory signalling, even after SARS-CoV-2 has been cleared from the body. During long COVID/PASC, Ca2+-binding protein S100A8/A9 chronically stimulates TLR4/RAGE-signalling to induce chronic expression of IL-1b, IL-6 and TNFa. Secreted IL-6 binds to its IL-6R receptor on the surface of other cells and signals via STAT3 and C/EBPb for more S100A8/A9 expression. Secreted IL-1b binds to its receptor IL-1R on other cells, and signals via NFkB for more mRAGE and TLR4 expression. New S100A8/A9 can bind and activate cell-surface mRAGE and TLR4 to stimulate expression of more IL- 1b, IL-6 and TNFa. This process establishes a pathogenic pro-inflammatory TLR4/RAGE-loop: IL-1b + IL-6 > IL-1R + IL-6R > TLR4/mRAGE + S100A8/A9 > IL-1b + IL-6, which generates multi-organ inflammation that persists in the blood vessels, the brain, the liver, the heart, the kidneys, the gut and the musculo-skeletal system, and is responsible for all the complex pathologies associated with long COVID/PASC. Chronic expression of IL-1, IL-6 and TNFa is critical for the maintenance of the TLR4/RAGE-loop and persistence of long COVID/PASC. Ezrin peptides are inhibitors of IL-1, IL-6, IL-8 and TNFa expression, so are now being investigated as potential therapy for long COVID/PASC. There is preliminary anecdotal evidence of symptomatic relief (not confirmed yet by formal clinical trials) from a few long COVID/PASC patient volunteers, after treatment with ezrin peptide therapy. Keywords: long COVID; PASC; RAGE; TLR4; p38MAPK; IL-1b; IL-6; IL-8; TNFa; S100; S100A8/A9; AGE; HMGB1; ezrin peptide therapy; HEP-1; RepG3
Keywords: long COVID; PASC; RAGE; TLR4; p38MAPK; IL-1b; IL-6; IL-8; TNFa; S100; S100A8/A9; AGE; HMGB1; ezrin peptide therapy; HEP-1; RepG3
Last edited by a moderator: