Abstract
Chimeric antigen receptor (CAR) T cell therapy was recently proposed as a treatment for adults with B-cell-mediated autoimmune diseases (ADs) refractory to conventional immunomodulatory therapy. We present a case series of eight children with severe/refractory AD (four systemic lupus erythematosus, three dermatomyositis, one systemic sclerosis) treated at Ospedale Pediatrico Bambino Gesù, Rome, and University Hospital Erlangen with a single infusion of 1 × 106 kg−1point-of-care manufactured autologous CD19 CAR T cells (zorpocabtagene autoleucel), in a hospital exemption (HE) program. In Europe, the HE pathway offers the opportunity to treat patients with life-threatening or seriously debilitating disorders who lack valid therapeutic options, using an advanced therapy medicinal product (ATMP) authorized on a nonroutine, single-patient basis. In contrast to the ‘compassionate use’ pathway, the ATMP does not necessarily need to have undergone clinical trials or marketing authorization applications. Manufacturing was successful in all patients, yielding several drug product bags. Once infused after lymphodepletion, zorpocabtagene autoleucel cells expanded in vivo, promoting prompt B cell clearance. Grade 1 cytokine release syndrome was reported in six patients, and grade 1 immune effector cell-associated neurotoxicity syndrome was reported in one patient. Late-hematotoxicity was limited to grade 1 in two patients. All these adverse events were manageable and no severe infections occurred. With a median follow-up of 16.5 months (range = 9–24 months), all patients experienced a clinically substantial improvement/resolution of AD, as evidenced by reduction in disease activity scores and signs of reversal of organ damage. This improvement enabled sustained discontinuation of immunomodulators, even after B cell reconstitution. The activation of formal clinical trials enrolling children and adolescents is urgently needed to confirm these preliminary results and to assess the long-term safety of this approach.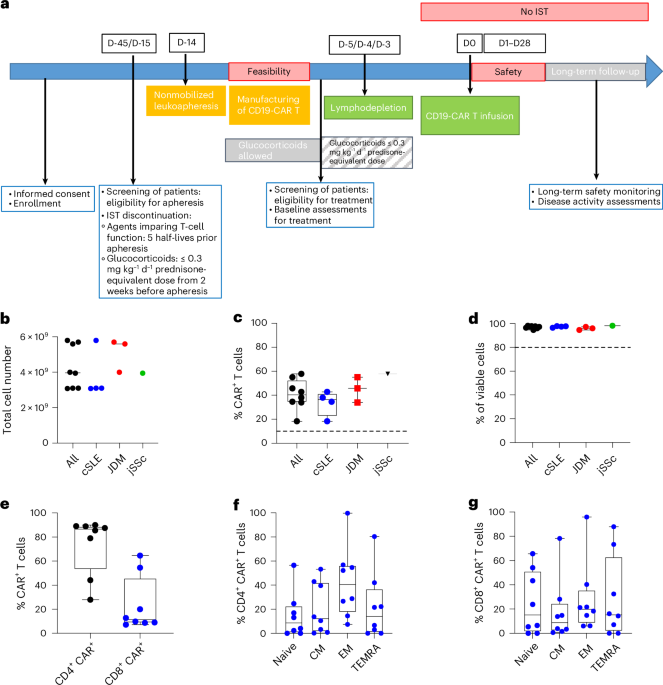
Anti-CD19 CAR T cells for pediatric patients with treatment-refractory autoimmune diseases - Nature Medicine
In a case series of eight pediatric patients with systemic lupus erythematosus, dermatomyositis or systemic sclerosis, autologous anti-CD19 CAR T cell therapy was feasible, safe and effective in reducing disease activity.
