hotblack
Senior Member (Voting Rights)
See post #8 for peer-reviewed version
------------------------------------------------
Virus-Induced Endothelial Senescence as a Cause and Driving Factor for ME/CFS and Long COVID: Mediated by a Dysfunctional Immune System
Massimo Nunes, Loren Kell, Anouk Slaghekke, Rob Wüst, Burtram Fielding, Douglas Kell, Etheresia Pretorius
Abstract
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and long COVID are two post-viral diseases, which share many common symptoms and pathophysiological alterations. Yet a mechanistic explanation of disease induction and maintenance is lacking. This hinders the discovery and implementation of biomarkers and treatment options, and ultimately the establishment of effective clinical resolution.
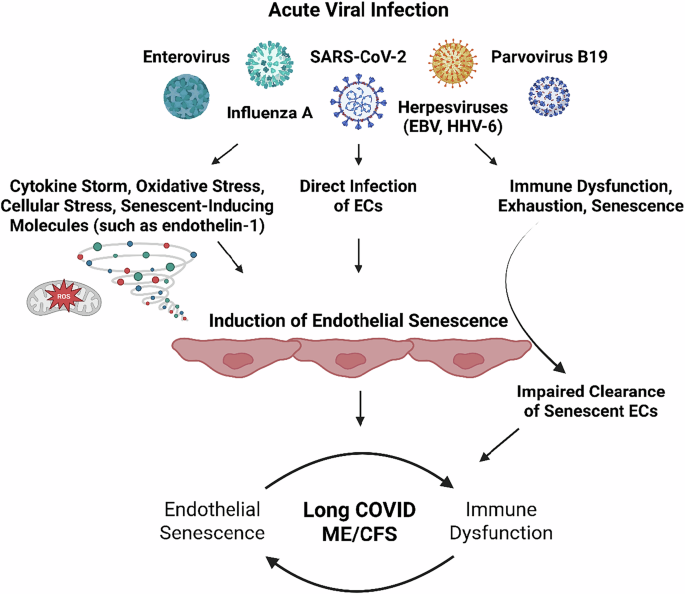
Here, we propose that acute viral infection results in (in)direct endothelial dysfunction and senescence, which at the blood-brain barrier, cerebral arteries, gastrointestinal tract, and skeletal muscle can explain symptoms. The endothelial senescence-associated secretory phenotype (SASP) is proinflammatory, pro-oxidative, procoagulant, primed for vasoconstriction, and characterized by impaired regulation of tissue repair, but also leads to dysregulated inflammatory processes. Immune abnormalities in ME/CFS and long COVID can account for the persistence of endothelial senescence long past the acute infection by preventing their clearance, thereby providing a mechanism for the chronic nature of ME/CFS and long COVID.
The systemic and tissue-specific effects of endothelial senescence can thus explain the multisystem involvement in and subtypes of ME/CFS and long COVID, including dysregulated blood flow and perfusion deficits. This can occur in all tissues, but especially the brain as evidenced by findings of reduced cerebral blood flow and impaired perfusion of various brain regions, post-exertional malaise (PEM), gastrointestinal disturbances, and fatigue. Paramount to this theory is the affected endothelium, and the bidirectional sustainment of immune abnormalities and endothelial senescence.
The recognition of endothelial cell dysfunction and senescence as a core element in the aetiology of both ME/CFS and Long COVID should aid in the establishment of effective biomarkers and treatment regimens.
Link (preprints.org) [not peer reviewed]
https://doi.org/10.20944/preprints202505.1875.v1
------------------------------------------------
Virus-Induced Endothelial Senescence as a Cause and Driving Factor for ME/CFS and Long COVID: Mediated by a Dysfunctional Immune System
Massimo Nunes, Loren Kell, Anouk Slaghekke, Rob Wüst, Burtram Fielding, Douglas Kell, Etheresia Pretorius
Abstract
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and long COVID are two post-viral diseases, which share many common symptoms and pathophysiological alterations. Yet a mechanistic explanation of disease induction and maintenance is lacking. This hinders the discovery and implementation of biomarkers and treatment options, and ultimately the establishment of effective clinical resolution.
Here, we propose that acute viral infection results in (in)direct endothelial dysfunction and senescence, which at the blood-brain barrier, cerebral arteries, gastrointestinal tract, and skeletal muscle can explain symptoms. The endothelial senescence-associated secretory phenotype (SASP) is proinflammatory, pro-oxidative, procoagulant, primed for vasoconstriction, and characterized by impaired regulation of tissue repair, but also leads to dysregulated inflammatory processes. Immune abnormalities in ME/CFS and long COVID can account for the persistence of endothelial senescence long past the acute infection by preventing their clearance, thereby providing a mechanism for the chronic nature of ME/CFS and long COVID.
The systemic and tissue-specific effects of endothelial senescence can thus explain the multisystem involvement in and subtypes of ME/CFS and long COVID, including dysregulated blood flow and perfusion deficits. This can occur in all tissues, but especially the brain as evidenced by findings of reduced cerebral blood flow and impaired perfusion of various brain regions, post-exertional malaise (PEM), gastrointestinal disturbances, and fatigue. Paramount to this theory is the affected endothelium, and the bidirectional sustainment of immune abnormalities and endothelial senescence.
The recognition of endothelial cell dysfunction and senescence as a core element in the aetiology of both ME/CFS and Long COVID should aid in the establishment of effective biomarkers and treatment regimens.
Link (preprints.org) [not peer reviewed]
https://doi.org/10.20944/preprints202505.1875.v1
Last edited by a moderator: