Now published - details at this post
Preprint detail below
***
https://www.authorea.com/users/6382...chronic-fatigue-syndrome-me-cfs-blood-samples
Bioimpedance spectroscopy characterization of osmotic stress processes in Myalgic Encephalomyelitis / Chronic Fatigue Syndrome (ME-CFS) blood samples
Abstract
Myalgic Encephalomyelitis / Chronic Fatigue Syndrome (ME/ CFS) is a disabling, chronic, multi-system and complex disease. Currently, there are no specific laboratory tests to directly [diagnose ME/CFS](https://www.cdc.gov/me-cfs/symptoms-diagnosis/diagnosis.html). In this work we study the use of impedance spectroscopy as a potential technique for the diagnosis of this disease. A specific device for the electrical characterization of peripheral blood mononuclear cells was designed and implemented. Impedance spectroscopy measurements in the range from 1 Hz to 500 MHz were made after osmotic stress of the samples with sodium chloride solution 1M. The evolution in time after the osmotic stress at two specific frequencies (1.36 kHz and 154 kHz) was analysed. The device showed its sensitivity to the presence of cells and the evolution of the osmotic process. Higher values of impedance were measured for 1.36 kHz in ME/CFS patients compared to control samples. Results help to further understand the relation of bioimpedance measurements with ME/CFS samples physical properties and osmotic processes.
Conclusions
In this work we study the use of impedance spectroscopy as a potential technique for the diagnosis of Myalgic Encephalomyelitis / Chronic Fatigue Syndrome (ME/ CFS). We have analysed peripheral blood mononuclear cells from four chronic fatigue patients and four healthy controls, after an osmotic stress of the samples with NaCl solution 1M.
A specific device for impedance measurement was designed and implemented, consisting on two interdigitated electrodes placed on a Petri dish, connected to an impedance analyser, obtaining measurements in the range from 1 Hz to 500 MHz. The device showed its good sensitivity to the presence of cells and the addition of NaCl to stress the cells, being an affordable option for a potential diagnostic solution in medical laboratories.
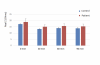
Real part and imaginary part of impedance were analysed for the whole duration of the experiment at two specific frequencies. At 1.36 kHz, higher values of impedance (both in the real part and the imaginary part, in absolute values) were measured in ME/CFS patients, in comparison with control individuals. As this frequency is located in the alpha dispersion region, the difference of impedance may be related to the effect of extra-cellular ions. At 154 kHz, no significant difference has been found between patient samples and control samples.
Preprint detail below
***
https://www.authorea.com/users/6382...chronic-fatigue-syndrome-me-cfs-blood-samples
Bioimpedance spectroscopy characterization of osmotic stress processes in Myalgic Encephalomyelitis / Chronic Fatigue Syndrome (ME-CFS) blood samples
Abstract
Myalgic Encephalomyelitis / Chronic Fatigue Syndrome (ME/ CFS) is a disabling, chronic, multi-system and complex disease. Currently, there are no specific laboratory tests to directly [diagnose ME/CFS](https://www.cdc.gov/me-cfs/symptoms-diagnosis/diagnosis.html). In this work we study the use of impedance spectroscopy as a potential technique for the diagnosis of this disease. A specific device for the electrical characterization of peripheral blood mononuclear cells was designed and implemented. Impedance spectroscopy measurements in the range from 1 Hz to 500 MHz were made after osmotic stress of the samples with sodium chloride solution 1M. The evolution in time after the osmotic stress at two specific frequencies (1.36 kHz and 154 kHz) was analysed. The device showed its sensitivity to the presence of cells and the evolution of the osmotic process. Higher values of impedance were measured for 1.36 kHz in ME/CFS patients compared to control samples. Results help to further understand the relation of bioimpedance measurements with ME/CFS samples physical properties and osmotic processes.
Conclusions
In this work we study the use of impedance spectroscopy as a potential technique for the diagnosis of Myalgic Encephalomyelitis / Chronic Fatigue Syndrome (ME/ CFS). We have analysed peripheral blood mononuclear cells from four chronic fatigue patients and four healthy controls, after an osmotic stress of the samples with NaCl solution 1M.
A specific device for impedance measurement was designed and implemented, consisting on two interdigitated electrodes placed on a Petri dish, connected to an impedance analyser, obtaining measurements in the range from 1 Hz to 500 MHz. The device showed its good sensitivity to the presence of cells and the addition of NaCl to stress the cells, being an affordable option for a potential diagnostic solution in medical laboratories.
Real part and imaginary part of impedance were analysed for the whole duration of the experiment at two specific frequencies. At 1.36 kHz, higher values of impedance (both in the real part and the imaginary part, in absolute values) were measured in ME/CFS patients, in comparison with control individuals. As this frequency is located in the alpha dispersion region, the difference of impedance may be related to the effect of extra-cellular ions. At 154 kHz, no significant difference has been found between patient samples and control samples.
Last edited by a moderator: