jnmaciuch
Senior Member (Voting Rights)
Chronic CXCL10 alters neuronal properties in rat hippocampal culture
Abstract
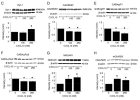
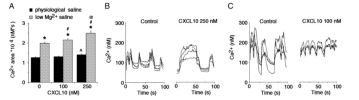
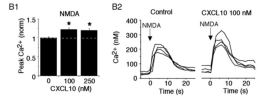
The chemokine CXCL10 is expressed in the central nervous system (CNS) during neuroinflammatory conditions. Neurons express CXCR3, the receptor for CXCL10, and neuronal function has been shown to be altered by acute exposure to CXCL10. Little is known about the effects of chronic exposure to CXCL10 on neuronal function. Results from our studies show that chronic exposure of cultured rat hippocampal neurons to CXCL10 results in altered levels of protein for GABA and glutamate receptors and altered synaptic network activity. These effects of CXCL10 may contribute to altered CNS function that occurs in some chronic neuroinflammatory conditions.
Web | Journal of Neuroimmunology
https://doi.org/10.1016/j.jneuroim.2008.12.007 (Paywalled)
Cho, Jungsook; Nelson, Thomas E.; Bajova, Hilda; Gruol, Donna L.
Abstract
The chemokine CXCL10 is expressed in the central nervous system (CNS) during neuroinflammatory conditions. Neurons express CXCR3, the receptor for CXCL10, and neuronal function has been shown to be altered by acute exposure to CXCL10. Little is known about the effects of chronic exposure to CXCL10 on neuronal function. Results from our studies show that chronic exposure of cultured rat hippocampal neurons to CXCL10 results in altered levels of protein for GABA and glutamate receptors and altered synaptic network activity. These effects of CXCL10 may contribute to altered CNS function that occurs in some chronic neuroinflammatory conditions.
Web | Journal of Neuroimmunology
https://doi.org/10.1016/j.jneuroim.2008.12.007 (Paywalled)