So the key finding from the Mendus study is
no significant improvement was observed for the ME/CFS group above what could be explained by placebo effect.
The study had two arms - one was a blinded crossover study of 51 people with ME/CFS, people were provided a 6 week supply of 20 mg capsules (one a day) without knowing whether it was MitoQ or a placebo and then later received a 6 week supply of the other. In the second arm, 43 people with ME/CFS bought their own MitoQ - (the commercial form with 5 mg capsules and guidelines suggesting a dosage of 10 mg/day). Both sets of participants completed the same surveys and online tests.
Of course, with this being an online study, we can't know for sure if the participants all had ME/CFS. The study was supported by the company producing MitoQ; they provided the free supply and they paid one of the authors to do the analysis.
Participants completed online assessments of fatigue, pain, sleep quality, mental clarity, gastrointestinal issues, depression and overall wellbeing, as well as an assessment of how active they had been. These were subjective assessments from 0 to 10, and were to cover the previous week. Participants also did three online cognitive tests.
Participants in the blinded study therefore were in the study for 2 x 6weeks. Participants in the unblinded study could provide data up until the 90 day mark.
Results
Blinded study: No effects were observed for the MitoQ group on
any measures above that observed in the placebo group.
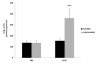
Open label study: Significant (indeed, large) positive effects were observed for many of the measures including decreased pain and increased energy, sleep quality, mental clarity, activity, well being and verbal reasoning. Many of these effects were observed at 6-weeks and continued to grow at two follow-up points of 2 and 3 months. At 6 weeks, energy was reported to have increased by an average of 26% (versus both the blinded placebo and the blinded MitoQ participants reporting decreases in energy at 6 weeks). This is despite the open dosage being probably half of what the blinded study participants were taking.
It's certainly interesting, as an example of how easy it is for people to convince themselves that something is helping.
(This study also had a fibromyalgia blinded arm - some positive effects were found for this. I haven't looked at those. But, in the ME/CFS study, the mean pain rating on a scale from 0 to 10 increased slightly from about 3.7 to 4 during the 6 weeks of MitoQ.)
There was some discussion about the possibility that the higher dose in the blinded study caused sleep problems and nausea.
6 people discontinued the blinded study due to side effects (it's not clear if this includes the fibromyalgia study) - but of these 6, 5 were taking the placebo.
The authors conclude:
It is noteworthy here to point out that the only positive effects we are aware of, concerning Q10 treatment of ME/CFS, were confounded by co-administration of NADH <referencing the Castro-Marrero team's work>. Thus, to date the literature is quite consistent, suggesting Q10 (or similar products such as MitoQ) are not effective, alone, for ME/CFS while they have repeatedly been shown to aid fibromyalgia.