Could you quote the relevant part? I can only see the abstract.They didn’t observe different frequencies of B cell infiltration between their EBV and control conditions.
- Home
- Forums
- Other news and research
- Other news and research
- 'Conditions related to ME/CFS' news and research
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
EBV induces CNS homing of B cells attracting inflammatory T cells 2025 Laderach et al.
- Thread starter Jaybee00
- Start date
jnmaciuch
Senior Member (Voting Rights)
Ah sorry I didn't realize it was paywalled. I'm spoiled by institutional accessCould you quote the relevant part? I can only see the abstract.
To investigate which leukocytes carry EBV to the CNS, we analysed the immune cell infiltrates from perfused brains of EBV-infected humanized BRGS-A2DR2 mice by flow cytometry. Although the frequency of CD19+ B cells in the brain did not differ between EBV-infected and PBS mice, (Extended Data Fig. 1g), we observed noticeable changes in the CD3+ T cell compartment in the brains of EBV-infected mice.
We next explored whether primary EBV infection can lead to the expansion and enrichment of ABCs in the CNS. Although CD11c is often associated with the ABC phenotype, we found that its expression on CD19+ B cells was not increased following EBV infection (Extended Data Fig. 2a,b), nor did we observe an upregulation of T-bet expression on CD11c+CD19+ cells in the spleen or brain, or an increase in their total numbers after EBV infection (Extended Data Fig. 2c,d). However, we observed significantly elevated frequencies of T-bet+CXCR3+ double-positive CD19+ B cells in the blood, spleen and brain following EBV infection (Fig. 2a–c). In addition, a positive correlation was found between total number of T-bet+CXCR3+ B cells in the spleen and splenic viral loads, indicating that infection drives T-bet+CXCR3+ B cell expansion (Fig. 2d). T-bet+CXCR3+ B cells expressed significantly higher frequencies of CD11c in the blood and spleen but not in the brain than the T-bet−CXCR3− B cell population. However, this difference was independent of EBV infection status and could also be observed in uninfected mice (Extended Data Fig. 2e,f).
So as has been shown in many other mouse studies, T cells and B cells get into the brain just fine even in healthy conditions. What seems to be relevant in disease is something different that happens once those B cells are already in the brain, which seems to be mediated by EBV and CXCR3 in an unknown way.
jnmaciuch
Senior Member (Voting Rights)
Next, to assess whether these T-bet+CXCR3+ B cells interact with T cell populations, we performed multiplex immunofluorescence on EBV-infected spleens, which revealed that T-bet+CXCR3+ B cells typically colocalized with CD4+ and CD8+ T cells (Fig. 2e). Characterizing cellular neighbourhoods revealed that T-bet+CXCR3+ B cells were located in areas that were enriched in activated HLA-DR+CD4+ T cells (Fig. 2f). Indeed, a higher frequency of activated HLA-DR+CD4+ T cells was found within the 25 nearest cells to T-bet+CXCR3+ B cells than other B cells (Fig. 2g)
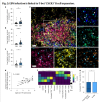
Actually, this is not very convincing. They're claiming that Fig. 2G supports the idea that this particular subset of co-localizes with activated CD4+ T-cells. And I have no idea how Fig. 2F is supposed to support the claim that "T-bet+CXCR3+ B cells were located in areas that were enriched in activated HLA-DR+CD4+ T cells."
Legend states:
The enrichment doesn't look higher for T-bet+CXCR3+ B cells than for any other B cell subset.f, Quantification of colocalization by neighbourhood analysis identified five neighbourhoods with the indicated cellular contents and neighbourhood size. The z-scores of cellular abundances per neighbourhood are displayed. DC, dentritic cell. g, Frequencies of activated (HLA-DR+) CD4+ T cells among the 25 nearest cells to T-bet+CXCR3+ B cells versus other B cells (P = 0.033; n = 2,568 B cells and n = 633 T-bet+CXCR3+ B cells; microenvironment (ME) of 1 independent experiment). The boxplots define the median value (centre), the interquartile range (25th and 75th percentiles) as the boundaries of the box, and whiskers extend to 1.5 times the 25th and 75th percentiles. Outliers, which lie outside the whiskers, are displayed as individual points. Note that the median value of B cells is 0. Panels
I guess this is why you have to go right to the figures and assume the abstract is lying to you.
That's interesting. The story from the other study is so nice though. Plenty of antibodies to other pathogens in CSF, but barely any to EBV, might suggest that primarily EBV-infected cells (that can't make EBV antibodies) are getting in.So as has been shown in many other mouse studies, T cells and B cells get into the brain just fine even in healthy conditions.
(Also Jonathan said B cells that make EBV antibodies can't support an EBV infection, but do we know that for certain?)
Edit: I mean, maybe that's what is unique about MS? In healthy people, a few of all B cells can get in. But in MS, EBV-infected cells are even more capable of getting in to the brain for some reason in problematic quantities.
Last edited:
jnmaciuch
Senior Member (Voting Rights)
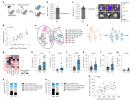
Figure 1 seems to have the only convincing graphs, which show higher proportions of HLA-DR+ T cells in the brain in EBV-infected mice.
I'm sorry, reading the rest of the paper, I truly do not think they support what the title of the paper claims. They confirm already known findings that CXCR3 expression on B cells seems to increase their migration to both the brain and spleen. Then they claim that these graphs from Fig 4 prove that T-bet expression drives T cell migration (and the text says that there was no significant difference in migration for CD8 T cells):
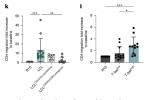
And then they show that rituximab reduces the frequency of some specific subsets of activated T cells in the brain.
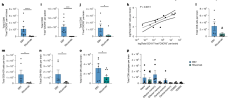
With high variability between their replications. So maybe overall these results slightly support the idea that if you kill off all B cells during EBV, you don't get as much migration of certain CD8+ subsets in the brain. Maybe
jnmaciuch
Senior Member (Voting Rights)
Perhaps! Unfortunately this study doesn't provide much evidence for that one way or another.Edit: I mean, maybe that's what is unique about MS? In healthy people, a few of all B cells can get in. But in MS, EBV-infected cells are even more capable of getting in to the brain for some reason in problematic quantities.
jnmaciuch
Senior Member (Voting Rights)
That's interesting. The story from the other study is so nice though. Plenty of antibodies to other pathogens in CSF, but barely any to EBV, might suggest that primarily EBV-infected cells (that can't make EBV antibodies) are getting in.
(Also Jonathan said B cells that make EBV antibodies can't support an EBV infection, but do we know that for certain?)
Actually I think the shreds of evidence this paper provides might hint more towards the opposite story. The B cells detected in the brain in EBV were oligoclonally expanded and shared with the spleen. And those clones showed reactivity to EBV proteins, compared to no reactivity in controls. They also said that the majority of T-bet+CXCR3+ B cells were EBV-infected, and that these were also clonally expanded.
There's some gray area here since they didn't confirm reactivity to EBV and infection in the same cells (the assays for that might be incompatible), but I think it's a pretty safe bet that EBV infected cells can still make EBV-specific antibodies, and in fact this is one strategy EBV uses to increase its numbers through clonal expansion (like HIV does in T cells).
Sorry, my brain is too mushy to follow this very well. If they didn't confirm it's not the same cells, then how can you know it's EBV-infected cells reacting to EBV?Actually I think the shreds of evidence this paper provides might hint more towards the opposite story. The B cells detected in the brain in EBV were oligoclonally expanded and shared with the spleen. And those clones showed reactivity to EBV proteins, compared to no reactivity in controls. They also said that the majority of T-bet+CXCR3+ B cells were EBV-infected, and that these were also clonally expanded.
There's some gray area here since they didn't confirm reactivity to EBV and infection in the same cells (the assays for that might be incompatible), but I think it's a pretty safe bet that EBV infected cells can still make EBV-specific antibodies, and in fact this is one strategy EBV uses to increase its numbers through clonal expansion (like HIV does in T cells).
And for the "strategy EBV uses" part, it sounds like you're saying it's settled then that they can make EBV antibodies?
Jonathan Edwards
Senior Member (Voting Rights)
So the story this presents is that B cells get into the brain regardless
The caveat being that this is a vey strange system with presumably mouse lectins on endothelial cells that may allow in humanised immune cells in a way that does not occur in the human. I doubt that normal human brain tissue has many B cells in it.
Edit: Google says very low numbers and mostly in meninges.
Last edited:
jnmaciuch
Senior Member (Voting Rights)
Because the clonal expansion is triggered by recognition of an antigen, and the antigen was EBV. They cultured B cells with EBV and showed that the T-bet+CXCR3+ subset, which was really the only subset enriched in the brain, clonally expanded more than other B cell subsets. And they know that these cells were infected because they became immortalized, which is what happens when EBV establishes latency.Sorry, my brain is too mushy to follow this very well. If they didn't confirm it's not the same cells, then how can you know it's EBV-infected cells reacting to EBV?
And for the "strategy EBV uses" part, it sounds like you're saying it's settled then that they can make EBV antibodies?
Yes, mice are not humans and its a weird system, which means there might be nothing mechanistic in this study that is relevant to human MS.The caveat being that this is a vey strange system with presumably mouse lectins on endothelial cells that may allow in humanised immune cells in a way that does not occur in the human. I doubt that normal human brain tissue has many B cells in it.
Jonathan Edwards
Senior Member (Voting Rights)
Yes, mice are not humans and its a weird system, which means there might be nothing mechanistic in this study that is relevant to human MS.
But the differential effect on a subset may still be informative?
There are a bunch of arguments around this but the study lends some support to the idea that the real question in MS is why some peculiar subset of B cells survive and mature into plasma cells in brain and, as expected, bring in some T cells that may be of no great interest. If we accept that EBV is necessary then a pointer to this subset expressing a particular surface ligand might be helpful.
I don't know the recent data but the first rituximab study in MS pretty much showed blocking of new lesions while B cells were depleted. If a more selective B cell depletion approach is all that is needed that could be very important = allowing my neighbour to have continuing therapy without worrying about hypogammaglobulinaemia.
jnmaciuch
Senior Member (Voting Rights)
Sure maybe. But this study doesn’t lend any support whatsoever for the idea of a unique mechanism allowing B cells to get into the brain where they wouldn’t otherwise. Increased infiltration was not a feature of the B cell subsets studied here. Which is what my original comment you quoted was saying.But the differential effect on a subset may still be informative?
There are a bunch of arguments around this but the study lends some support to the idea that the real question in MS is why some peculiar subset of B cells survive and mature into plasma cells in brain and, as expected, bring in some T cells that may be of no great interest. If we accept that EBV is necessary then a pointer to this subset expressing a particular surface ligand might be helpful.
I don't know the recent data but the first rituximab study in MS pretty much showed blocking of new lesions while B cells were depleted. If a more selective B cell depletion approach is all that is needed that could be very important = allowing my neighbour to have continuing therapy without worrying about hypogammaglobulinaemia.
If you’re saying that the weirdness of this model is what allows B cells into the brain even in healthy conditions in a way that doesn’t happen in human brains, then the same confounders apply to anything regarding B cell infiltration in the EBV condition.
So the story this presents is that B cells get into the brain regardless, often at low quantities, but something about being EBV-infected leads a certain subtype of B cells to recruit HLA-DR+ T cells, which then leads to the brain damage.
So as has been shown in many other mouse studies, T cells and B cells get into the brain just fine even in healthy conditions. What seems to be relevant in disease is something different that happens once those B cells are already in the brain, which seems to be mediated by EBV and CXCR3 in an unknown way.
this study doesn’t lend any support whatsoever for the idea of a unique mechanism allowing B cells to get into the brain where they wouldn’t otherwise. Increased infiltration was not a feature of the B cell subsets studied here.
See Myelin antigen capture in the CNS by B cells expressing EBV latent membrane protein 1 leads to demyelinating lesion formation (2026) —
We show (1) that in animal models naive, myelin-reactive B cells enter the CNS during infections, (2) that they can capture antigen directly from previously healthy myelin, and (3) that the EBV protein LMP1 can rescue them from the normal tolerance mechanism of AICD.
In summary, we present a model of lesion initiation in MS centered on EBV-infected, myelin-reactive B cells in the context of non-autoimmune-mediated immune cell infiltration. This model explains how certain prodromes such as CNS infection and head trauma can increase the risk of MS, and it strengthens the rationale for a clinical vaccination trial aimed at preventing or ameliorating EBV infection in adolescents to reduce the risk of MS.
jnmaciuch
Senior Member (Voting Rights)
Interesting, that does seem to address some of the unanswered questions in this study.
