Chandelier
Senior Member (Voting Rights)
Effects of Cacao Flavonoids in Long COVID-19 Patients with Chronic Fatigue: FLALOC, a Placebo-Controlled Randomized Clinical Trial
Abstract
Background: In the context of long COVID, persistent fatigue is among the most prevalent symptoms that can develop after SARS-CoV-2 infection.
Mitochondrial myopathy and endothelial dysfunction, which are triggers of inflammation, have emerged as prominent causes of long COVID-induced fatigue. Interestingly, the intake of flavanols, particularly (−)-epicatechin (EC), has been associated with the positive modulation of endothelial and mitochondrial structure and function.
Methods: In this work, we conducted a randomized, double-blind, placebo-controlled clinical trial to determine whether an EC-enriched supplement (ECES) improves plasma markers of inflammation, endothelial structure, and fatigue-related endpoints in patients with long COVID-19.
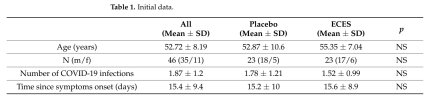
Results: The study included 46 subjects (mean age 52 years) who were instructed to consume two capsules/day for 90 days of either ECES (n = 23) or placebo (n = 23).
Endpoints assessed included mean changes in plasma inflammatory markers (IL-1β, IL-6, and TNF-α) and endothelial dysfunction markers (syndecan-1), handgrip strength, fatigue scale, and quality of life (QoL).
The results showed significant improvements in the ECES group for inflammatory markers, syndecan-1, and fatigue compared with the placebo group.
Conclusions: The results yield intriguing positive findings for EC and open a new avenue for treating long COVID.
Web | DOI | Journal of Clinical Medicine
Munguía, Levy; Silva, Selene; Villarreal, Francisco; Nájera, Nayelli; Ceballos, Guillermo
Abstract
Background: In the context of long COVID, persistent fatigue is among the most prevalent symptoms that can develop after SARS-CoV-2 infection.
Mitochondrial myopathy and endothelial dysfunction, which are triggers of inflammation, have emerged as prominent causes of long COVID-induced fatigue. Interestingly, the intake of flavanols, particularly (−)-epicatechin (EC), has been associated with the positive modulation of endothelial and mitochondrial structure and function.
Methods: In this work, we conducted a randomized, double-blind, placebo-controlled clinical trial to determine whether an EC-enriched supplement (ECES) improves plasma markers of inflammation, endothelial structure, and fatigue-related endpoints in patients with long COVID-19.
Results: The study included 46 subjects (mean age 52 years) who were instructed to consume two capsules/day for 90 days of either ECES (n = 23) or placebo (n = 23).
Endpoints assessed included mean changes in plasma inflammatory markers (IL-1β, IL-6, and TNF-α) and endothelial dysfunction markers (syndecan-1), handgrip strength, fatigue scale, and quality of life (QoL).
The results showed significant improvements in the ECES group for inflammatory markers, syndecan-1, and fatigue compared with the placebo group.
Conclusions: The results yield intriguing positive findings for EC and open a new avenue for treating long COVID.
Web | DOI | Journal of Clinical Medicine