Dolphin
Senior Member (Voting Rights)
Poster abstract, see post #4 for full paper.
https://www.neurology.org/doi/abs/10.1212/WNL.0000000000204829
SLEEP 3
April 9, 2024
Free Access
Actigraphic and Genetic Characterization of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Phenotypes in the UK Biobank (P10-9.007)
Patrick Liu, David Raizen, Carsten Skarke, Thomas Brooks, and Ron Anafi
AUTHORS INFO & AFFILIATIONS
April 9, 2024 issue
102 (17_supplement_1)
https://doi.org/10.1212/WNL.0000000000204829
Abstract
Objective:
Patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) often experience debilitating fatigue and autonomic dysregulation, yet objective measurements of these symptoms are limited. This study utilized actigraphic data from the United Kingdom Biobank (UKBB) to investigate (1) reduced activity in those with CFS, (2) decreased amplitudes of daily temperature rhythms as a potential indicator of autonomic dysregulation, and (3) the impact of specific single nucleotide polymorphisms (SNPs) associated with CFS on these actigraphic parameters.
Background:
ME/CFS is a complex and poorly understood condition characterized by profound fatigue, postural orthostasis, and temperature dysregulation. Objective metrics reflecting these fatigue-related symptoms are scarce. Previous research explored small-scale actigraphic analyses, shedding light on movement and temperature patterns in CFS, but large-scale investigations remain limited. Genetic factors have also emerged as potential contributors to CFS risk, although how they affect phenotypic manifestations remains unclear.
Design/Methods:
Actigraphic data from the UKBB were analyzed to compare those with CFS (n = 295) to controls (n = 63,133). Movement parameters, acceleration amplitudes, and temperature amplitudes were assessed. Additionally, the impact of specific SNPs associated with CFS on actigraphic measurements and subjective fatigue experiences was examined.
Results:
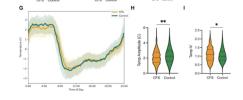
In addition to profound fatigue, those with CFS exhibited significantly reduced overall movement (Cohen’s d = −0.220, p-value = 2.42 × 10–15), lower acceleration amplitudes (Cohen’s d = −0.377, p-value = 1.74 × 10−6), and decreased temperature amplitudes (Cohen’s d = −0.173, p-value = 0.002) compared to controls. Furthermore, certain SNPs associated with CFS were found to significantly influence both actigraphic measurements and subjective fatigue experiences.
Conclusions:
This study provides valuable insights into the objective characterization of CFS using actigraphy, shedding light on the interaction between genetics and symptomatology in CFS. The findings offer avenues for further research into the pathophysiology of CFS and may contribute to a better understanding of fatigue-related conditions in general.
Disclosure: Dr. Liu has nothing to disclose. Dr. Raizen has nothing to disclose. Dr. Skarke has nothing to disclose. Dr. Brooks has nothing to disclose. Dr. Anafi has nothing to disclose.
https://www.neurology.org/doi/abs/10.1212/WNL.0000000000204829
SLEEP 3
April 9, 2024
Free Access
Actigraphic and Genetic Characterization of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Phenotypes in the UK Biobank (P10-9.007)
Patrick Liu, David Raizen, Carsten Skarke, Thomas Brooks, and Ron Anafi
AUTHORS INFO & AFFILIATIONS
April 9, 2024 issue
102 (17_supplement_1)
https://doi.org/10.1212/WNL.0000000000204829
Abstract
Objective:
Patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) often experience debilitating fatigue and autonomic dysregulation, yet objective measurements of these symptoms are limited. This study utilized actigraphic data from the United Kingdom Biobank (UKBB) to investigate (1) reduced activity in those with CFS, (2) decreased amplitudes of daily temperature rhythms as a potential indicator of autonomic dysregulation, and (3) the impact of specific single nucleotide polymorphisms (SNPs) associated with CFS on these actigraphic parameters.
Background:
ME/CFS is a complex and poorly understood condition characterized by profound fatigue, postural orthostasis, and temperature dysregulation. Objective metrics reflecting these fatigue-related symptoms are scarce. Previous research explored small-scale actigraphic analyses, shedding light on movement and temperature patterns in CFS, but large-scale investigations remain limited. Genetic factors have also emerged as potential contributors to CFS risk, although how they affect phenotypic manifestations remains unclear.
Design/Methods:
Actigraphic data from the UKBB were analyzed to compare those with CFS (n = 295) to controls (n = 63,133). Movement parameters, acceleration amplitudes, and temperature amplitudes were assessed. Additionally, the impact of specific SNPs associated with CFS on actigraphic measurements and subjective fatigue experiences was examined.
Results:
In addition to profound fatigue, those with CFS exhibited significantly reduced overall movement (Cohen’s d = −0.220, p-value = 2.42 × 10–15), lower acceleration amplitudes (Cohen’s d = −0.377, p-value = 1.74 × 10−6), and decreased temperature amplitudes (Cohen’s d = −0.173, p-value = 0.002) compared to controls. Furthermore, certain SNPs associated with CFS were found to significantly influence both actigraphic measurements and subjective fatigue experiences.
Conclusions:
This study provides valuable insights into the objective characterization of CFS using actigraphy, shedding light on the interaction between genetics and symptomatology in CFS. The findings offer avenues for further research into the pathophysiology of CFS and may contribute to a better understanding of fatigue-related conditions in general.
Disclosure: Dr. Liu has nothing to disclose. Dr. Raizen has nothing to disclose. Dr. Skarke has nothing to disclose. Dr. Brooks has nothing to disclose. Dr. Anafi has nothing to disclose.
Last edited by a moderator: