Genome-wide study of somatic symptom and related disorders identifies novel genomic loci and map genetic architecture
[Line breaks added]
Abstract
Somatic symptom and related disorders (SSRD) are characterized by a mixture of neurological and psychiatric features and include functional neurological (FND) and somatic symptom disorders (SomD). While these complex neuropsychiatric disorders show evidence of genetic susceptibility, there are no genome-wide association studies (GWAS) of SSRD, and the heritability is unknown.
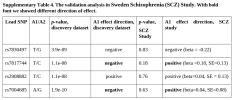
We did a GWAS of a total of 22,203 patients with SSRD, and 1,831,107 controls of European ancestry. We identified one genome-wide significant locus (chromosome 8:65565084) in SSRD, and one additional locus (chromosome 16:49074278) in the SomD subgroup (n cases = 18,536).
The observed-scale SNP heritability was estimated to be 7.3 % for SSRD, 15.7 % for FND and 7.7 % for SomD. FND and SomD were strongly genetically correlated (rg=0.94, SE=0.11, p=3.9E-18). SSRD showed significant genetic correlation with psychiatric disorders (highest with anxiety, post-traumatic stress disorders, depression, rg=0.3- 0.8), neurological disorders (migraine, chronic pain, rg=0.4-0.6) and immune-related diseases (rg=0.2-0.3).
Functional follow-up analysis of SSRD loci implicated the genes CYP7B1, BHLHE22, and CBLN1, which are involved in metabolic and brain-related processes, suggesting common underlying pathways.
We identified genomic loci associations with SSRD and showed strong genetic correlation between FND and SomD and with neurological and psychiatric disorders, as well as immune-related diseases. The current findings highlight shared underlying pathophysiological processes between SSRD diagnostic categories.
Web | PDF | Preprint: MedRxiv | Open Access
Vera Fominykh, Piotr Jaholkowski, Alexey A. Shadrin, Elise Koch, Laura B. Luitva, Dorte H. Mikkelsen, Mischa Lundberg, Ole Birger Pedersen, Sisse Rye Ostrowski, Christian Erikstrup, Maria Didriksen, Christina Mikkelsen, Erik Sørensen, Henrik Ullum, Mie Topholm Bruun, Bitten Aagaard, Kaarina Kowalec, Robert Karlsson, Håkan Karlsson, Christina Dalman, Pravesh Parekh, Viktoria Birkenæs, Yury Seliverstov, Bernhard Landwehrmeyer, Ida E. Sønderby, DBDS genetic consortium, Estonian Biobank research team, Olav B. Smeland, Kevin S. O’Connell, Lu Yi, Patrick F. Sullivan, Thomas M. Werge, Lili Milani, Ole A. Andreassen
[Line breaks added]
Abstract
Somatic symptom and related disorders (SSRD) are characterized by a mixture of neurological and psychiatric features and include functional neurological (FND) and somatic symptom disorders (SomD). While these complex neuropsychiatric disorders show evidence of genetic susceptibility, there are no genome-wide association studies (GWAS) of SSRD, and the heritability is unknown.
We did a GWAS of a total of 22,203 patients with SSRD, and 1,831,107 controls of European ancestry. We identified one genome-wide significant locus (chromosome 8:65565084) in SSRD, and one additional locus (chromosome 16:49074278) in the SomD subgroup (n cases = 18,536).
The observed-scale SNP heritability was estimated to be 7.3 % for SSRD, 15.7 % for FND and 7.7 % for SomD. FND and SomD were strongly genetically correlated (rg=0.94, SE=0.11, p=3.9E-18). SSRD showed significant genetic correlation with psychiatric disorders (highest with anxiety, post-traumatic stress disorders, depression, rg=0.3- 0.8), neurological disorders (migraine, chronic pain, rg=0.4-0.6) and immune-related diseases (rg=0.2-0.3).
Functional follow-up analysis of SSRD loci implicated the genes CYP7B1, BHLHE22, and CBLN1, which are involved in metabolic and brain-related processes, suggesting common underlying pathways.
We identified genomic loci associations with SSRD and showed strong genetic correlation between FND and SomD and with neurological and psychiatric disorders, as well as immune-related diseases. The current findings highlight shared underlying pathophysiological processes between SSRD diagnostic categories.
Web | PDF | Preprint: MedRxiv | Open Access