RaviHVJ
Senior Member (Voting Rights)
Abstract
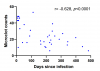
Outcomes following SARS-CoV-2 infection are variable; whilst the majority of patients recover without serious complications, a subset of patients develop prolonged illness termed Long COVID or post-acute sequelae of SARS-CoV-2 infection (PASC). The pathophysiology underlying Long COVID remains unclear but appears to involve multiple mechanisms including persistent inflammation, coagulopathy, autoimmunity, and organ damage. Studies suggest that microclots, also known as fibrinaloids, play a role in Long COVID. In this context, we developed a method to quantify microclots and investigated the relationship between microclot counts and Long COVID. We show that as a cohort, platelet-poor plasma from Long COVID samples had a higher microclot count compared to control groups but retained a wide distribution of counts. Recent COVID-19 infections were also seen to be associated with microclot counts higher than the control groups and equivalent to the Long COVID cohort, with a subsequent time-dependent reduction of counts. Our findings suggest that microclots could be a potential biomarker of disease and/or a treatment target in some Long COVID patients.
https://www.medrxiv.org/content/10.1101/2024.04.04.24305318v1
(This is an attempted replication of the microclots finding by a UK team)
Outcomes following SARS-CoV-2 infection are variable; whilst the majority of patients recover without serious complications, a subset of patients develop prolonged illness termed Long COVID or post-acute sequelae of SARS-CoV-2 infection (PASC). The pathophysiology underlying Long COVID remains unclear but appears to involve multiple mechanisms including persistent inflammation, coagulopathy, autoimmunity, and organ damage. Studies suggest that microclots, also known as fibrinaloids, play a role in Long COVID. In this context, we developed a method to quantify microclots and investigated the relationship between microclot counts and Long COVID. We show that as a cohort, platelet-poor plasma from Long COVID samples had a higher microclot count compared to control groups but retained a wide distribution of counts. Recent COVID-19 infections were also seen to be associated with microclot counts higher than the control groups and equivalent to the Long COVID cohort, with a subsequent time-dependent reduction of counts. Our findings suggest that microclots could be a potential biomarker of disease and/or a treatment target in some Long COVID patients.
https://www.medrxiv.org/content/10.1101/2024.04.04.24305318v1
(This is an attempted replication of the microclots finding by a UK team)