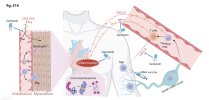
Inhibition of CXCL10 and IFN-γ ameliorates myocarditis in preclinical models of SARS-CoV-2 mRNA vaccination
Messenger RNA (mRNA) vaccines against SARS-CoV-2 are highly effective and were instrumental in curbing the COVID-19 pandemic. However, rare cases of noninfective myocarditis, particularly in young males and typically after the second dose, have been observed.
Here, we explore the mediators of this myocarditis to better understand and to enhance the safety of future mRNA vaccines. Through analysis of human plasma data and in vitro experiments with human macrophages and T cells, we identified increased C-X-C motif chemokine ligand 10 (CXCL10) and interferon-γ (IFN-γ) after exposure to BNT162b2 (Pfizer) or mRNA-1273 (Moderna).
Neutralization of CXCL10 and IFN-γ during the second dose (21 days after the first dose) reduced vaccine-induced cardiac injury in mice. Neutralization also reduced cardiac stress markers such as the release of N-terminal pro-B-type natriuretic peptide (NT-proBNP) and expression of inflammatory genes in human induced pluripotent stem cell (iPSC)–derived cardiac spheroids.
When exposed to these cytokines in vitro, human iPSC-derived cardiomyocytes (iPSC-CMs) exhibited impaired contractility, arrhythmogenicity, and proinflammatory gene expression patterns. Genistein, a phytoestrogen implicated in reducing cardiovascular inflammation, mitigated these effects in iPSC-CMs. In mice exposed to these cytokines or receiving BNT162b2 vaccination, genistein treatment reduced cardiac injury markers and attenuated infiltration of neutrophils and macrophages into the heart.
These findings implicate CXCL10–IFN-γ signaling as a contributor to myocardial injury in experimental models of mRNA vaccination and indicate that pharmacologic modulation, such as with genistein, may mitigate cytokine-driven injury.
EDITORS SUMMARY
The highly effective SARS-CoV-2 mRNA vaccines were essential for limiting the COVID-19 pandemic. In very rare cases, myocarditis has been reported, mostly in young males and usually after booster doses of the vaccines. Cao et al. used in vitro and mouse models to better understand the potential mechanism behind this myocarditis. C-X-C motif chemokine ligand 10 (CXCL10) and interferon-γ (IFN-γ) were identified as drivers of inflammation, and their neutralization reduced cardiac injury in a mouse model of two-dose vaccine exposure. Neutralization similarly reduced markers of cardiac injury in human induced pluripotent stem cell (iPSC)–derived cardiac spheroids.
Genistein, a compound with evidence for a role in reducing cardiovascular inflammation, similarly reduced cardiac injury markers and inflammation in mice exposed to CXCL10 and IFN-γ or to vaccines. Together, these data reveal a potential mechanism for the rare cases of myocarditis observed after SARS-CoV-2 mRNA vaccination.
Web | DOI | PDF | Science Translational Medicine | Paywall
Xu Cao; Amit Manhas; Yi-Ing Chen; Arianne Caudal; Gema Mondejar-Parreño; Wenjuan Zhu; Wenqiang Liu; Xiaohui Kong; Wenshu Zeng; Lichao Liu; Shane R Zhao; James W S Jahng; Paul J Utz; Kari C Nadeau; Masataka Nishiga; Joseph C Wu
Messenger RNA (mRNA) vaccines against SARS-CoV-2 are highly effective and were instrumental in curbing the COVID-19 pandemic. However, rare cases of noninfective myocarditis, particularly in young males and typically after the second dose, have been observed.
Here, we explore the mediators of this myocarditis to better understand and to enhance the safety of future mRNA vaccines. Through analysis of human plasma data and in vitro experiments with human macrophages and T cells, we identified increased C-X-C motif chemokine ligand 10 (CXCL10) and interferon-γ (IFN-γ) after exposure to BNT162b2 (Pfizer) or mRNA-1273 (Moderna).
Neutralization of CXCL10 and IFN-γ during the second dose (21 days after the first dose) reduced vaccine-induced cardiac injury in mice. Neutralization also reduced cardiac stress markers such as the release of N-terminal pro-B-type natriuretic peptide (NT-proBNP) and expression of inflammatory genes in human induced pluripotent stem cell (iPSC)–derived cardiac spheroids.
When exposed to these cytokines in vitro, human iPSC-derived cardiomyocytes (iPSC-CMs) exhibited impaired contractility, arrhythmogenicity, and proinflammatory gene expression patterns. Genistein, a phytoestrogen implicated in reducing cardiovascular inflammation, mitigated these effects in iPSC-CMs. In mice exposed to these cytokines or receiving BNT162b2 vaccination, genistein treatment reduced cardiac injury markers and attenuated infiltration of neutrophils and macrophages into the heart.
These findings implicate CXCL10–IFN-γ signaling as a contributor to myocardial injury in experimental models of mRNA vaccination and indicate that pharmacologic modulation, such as with genistein, may mitigate cytokine-driven injury.
EDITORS SUMMARY
The highly effective SARS-CoV-2 mRNA vaccines were essential for limiting the COVID-19 pandemic. In very rare cases, myocarditis has been reported, mostly in young males and usually after booster doses of the vaccines. Cao et al. used in vitro and mouse models to better understand the potential mechanism behind this myocarditis. C-X-C motif chemokine ligand 10 (CXCL10) and interferon-γ (IFN-γ) were identified as drivers of inflammation, and their neutralization reduced cardiac injury in a mouse model of two-dose vaccine exposure. Neutralization similarly reduced markers of cardiac injury in human induced pluripotent stem cell (iPSC)–derived cardiac spheroids.
Genistein, a compound with evidence for a role in reducing cardiovascular inflammation, similarly reduced cardiac injury markers and inflammation in mice exposed to CXCL10 and IFN-γ or to vaccines. Together, these data reveal a potential mechanism for the rare cases of myocarditis observed after SARS-CoV-2 mRNA vaccination.
Web | DOI | PDF | Science Translational Medicine | Paywall