Mij
Senior Member (Voting Rights)
Abstract
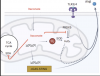
The immunoregulatory metabolite itaconate accumulates in innate immune cells upon Toll-like receptor stimulation. In response to macrophage activation by lipopolysaccharide, itaconate inhibits inflammasome activation and boosts type I interferon signalling; however, the molecular mechanism of this immunoregulation remains unclear. Here, we show that the enhancement of type I interferon secretion by itaconate depends on the inhibition of peroxiredoxin 5 and on mitochondrial reactive oxygen species.
We find that itaconate non-covalently inhibits peroxiredoxin 5, leading to the modulation of mitochondrial peroxide in activating macrophages. Through genetic manipulation, we confirm that peroxiredoxin 5 modulates type I interferon secretion in macrophages.
The non-electrophilic itaconate mimetic 2-methylsuccinate inhibits peroxiredoxin 5 and phenocopies immunoregulatory action of itaconate on type I interferon and inflammasome activation, providing further support for a non-covalent inhibition of peroxiredoxin 5 by itaconate.
LINK - paywall
Our work provides insight into the molecular mechanism of actions and biological rationale for the predominantly immune specification of itaconate.
The immunoregulatory metabolite itaconate accumulates in innate immune cells upon Toll-like receptor stimulation. In response to macrophage activation by lipopolysaccharide, itaconate inhibits inflammasome activation and boosts type I interferon signalling; however, the molecular mechanism of this immunoregulation remains unclear. Here, we show that the enhancement of type I interferon secretion by itaconate depends on the inhibition of peroxiredoxin 5 and on mitochondrial reactive oxygen species.
We find that itaconate non-covalently inhibits peroxiredoxin 5, leading to the modulation of mitochondrial peroxide in activating macrophages. Through genetic manipulation, we confirm that peroxiredoxin 5 modulates type I interferon secretion in macrophages.
The non-electrophilic itaconate mimetic 2-methylsuccinate inhibits peroxiredoxin 5 and phenocopies immunoregulatory action of itaconate on type I interferon and inflammasome activation, providing further support for a non-covalent inhibition of peroxiredoxin 5 by itaconate.
LINK - paywall
Our work provides insight into the molecular mechanism of actions and biological rationale for the predominantly immune specification of itaconate.
Last edited by a moderator: