LarsSG
Senior Member (Voting Rights)
Abstract
Background
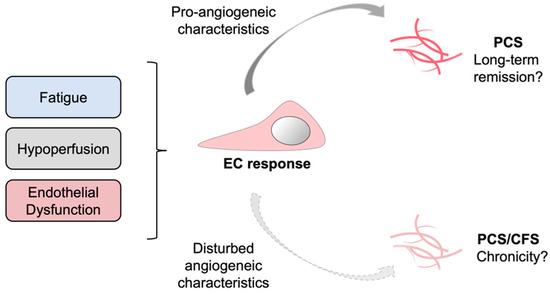
Recent studies have highlighted Coronavirus disease 2019 (COVID-19) as a multisystemic vascular disease. Up to 60% of the patients suffer from long-term sequelae and persistent symptoms even 6 months after the initial infection.
Methods
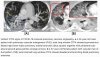
This prospective, observational study included 58 participants, 27 of whom were long COVID patients with persistent symptoms > 12 weeks after recovery from PCR-confirmed SARS-CoV-2 infection. Fifteen healthy volunteers and a historical cohort of critically ill COVID-19 patients (n = 16) served as controls. All participants underwent sublingual videomicroscopy using sidestream dark field imaging. A newly developed version of Glycocheck™ software was used to quantify vascular density, perfused boundary region (PBR-an inverse variable of endothelial glycocalyx dimensions), red blood cell velocity (VRBC) and the microvascular health score (MVHS™) in sublingual microvessels with diameters 4–25 µm.
Measurements and main results
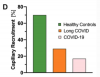
Although dimensions of the glycocalyx were comparable to those of healthy controls, a µm-precise analysis showed a significant decrease of vascular density, that exclusively affected very small capillaries (D5: − 45.16%; D6: − 35.60%; D7: − 22.79%). Plotting VRBC of capillaries and feed vessels showed that the number of capillaries perfused in long COVID patients was comparable to that of critically ill COVID-19 patients and did not respond adequately to local variations of tissue metabolic demand. MVHS was markedly reduced in the long COVID cohort (healthy 3.87 vs. long COVID 2.72 points; p = 0.002).
Conclusions
Our current data strongly suggest that COVID-19 leaves a persistent capillary rarefication even 18 months after infection. Whether, to what extent, and when the observed damage might be reversible remains unclear.
Full text
Background
Recent studies have highlighted Coronavirus disease 2019 (COVID-19) as a multisystemic vascular disease. Up to 60% of the patients suffer from long-term sequelae and persistent symptoms even 6 months after the initial infection.
Methods
This prospective, observational study included 58 participants, 27 of whom were long COVID patients with persistent symptoms > 12 weeks after recovery from PCR-confirmed SARS-CoV-2 infection. Fifteen healthy volunteers and a historical cohort of critically ill COVID-19 patients (n = 16) served as controls. All participants underwent sublingual videomicroscopy using sidestream dark field imaging. A newly developed version of Glycocheck™ software was used to quantify vascular density, perfused boundary region (PBR-an inverse variable of endothelial glycocalyx dimensions), red blood cell velocity (VRBC) and the microvascular health score (MVHS™) in sublingual microvessels with diameters 4–25 µm.
Measurements and main results
Although dimensions of the glycocalyx were comparable to those of healthy controls, a µm-precise analysis showed a significant decrease of vascular density, that exclusively affected very small capillaries (D5: − 45.16%; D6: − 35.60%; D7: − 22.79%). Plotting VRBC of capillaries and feed vessels showed that the number of capillaries perfused in long COVID patients was comparable to that of critically ill COVID-19 patients and did not respond adequately to local variations of tissue metabolic demand. MVHS was markedly reduced in the long COVID cohort (healthy 3.87 vs. long COVID 2.72 points; p = 0.002).
Conclusions
Our current data strongly suggest that COVID-19 leaves a persistent capillary rarefication even 18 months after infection. Whether, to what extent, and when the observed damage might be reversible remains unclear.
Full text