I was just looking at some of the first rituximab studies, and comparing them to the Daratumumab trial like some members were doing on the first pages here. They do have a point. The resemblance between the self reported results are eerie!! Sadly, no step counts were measured in the P1 and Ptwo as far as I can see. So the only thing we can go by is the difference in the P3 rituximab and Dara P1. Where the difference in my opinion is huge.
In my twenty five years of MECFS I have only had one brief remission, and just as I was starting to get worse during said remission I bought a Fitbit to track my daily steps.
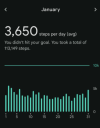
January represents my current daily steps.
View attachment 30426
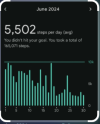
June is when I bought the Fitbit
View attachment 30427
You can see the point where my ´´remission`` really starts going downhill in the middle of June.
If the P1 trial results can be replicated then in my opinion it doesn`t just have massive biological implications for MECFS, but also has the implication that step count is an important outcome measure that should be used from now on to predict if the patients are actually genuinely improving. It isn`t perfect. But if the results can be replicated in the placebo trial then the step counts seem a better indicator of improvement? Depending on the sort of patients you include of course (For the majority of my time as mild I could easily do and probably did 10k steps a day, thus if I were included when I was mild in a study daily steps would be useless).
Thanks to Murph who has the comparison between the different rituximab trials and Daratumumab on the first page, if anyone is interested.