REGAIN: A Randomized Controlled Clinical Trial of Oxaloacetate for Improving the Symptoms of Long COVID
Suzanne D Vernon, Candace Rond, Jennifer Bell, Brindisi Butler, Sara Isolampi, Annaleah Otteson, Pearl Phalwane, Samantha Mower, Shad Roundy, David Kaufman, Alan Brian Cash, Lucinda Bateman
Background
Long COVID is characterized by fatigue, cognitive dysfunction, and other persistent symptoms. This randomized, double-blind, controlled trial evaluated the efficacy of oral oxaloacetate (OAA) in improving fatigue and cognitive function in adults with Long COVID.
Methods
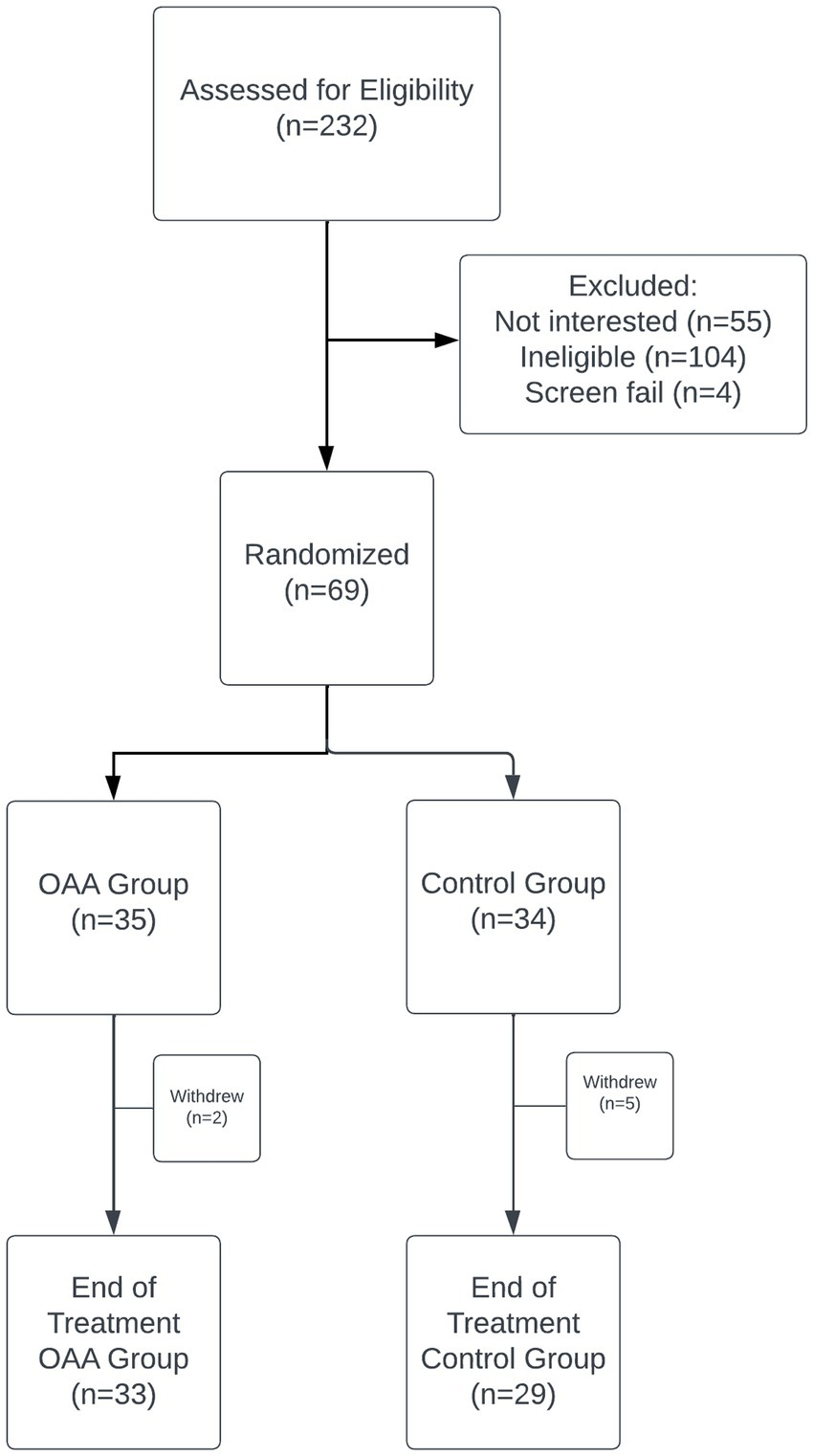
Sixty-nine participants were randomized to receive either 2,000 mg/day of OAA or control for 42 days. Primary outcome was fatigue reduction measured by the Chalder Fatigue Questionnaire (CFQ). Secondary and exploratory outcomes included the DePaul Symptom Questionnaire Short Form (DSQ-SF), health-related quality of life (RAND-36), cognitive function (DANA Brain Vital), and time upright (UP Time).
Results
No significant difference in CFQ fatigue reduction was observed between groups.
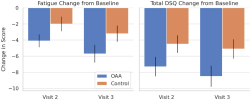
However, the OAA group showed significantly greater improvements in DSQ-SF fatigue and total symptom burden 21 days into the trial. Cognitive performance improved significantly in the OAA group, with strong correlations between symptom response and cognitive gains. OAA was well tolerated.
Conclusions
OAA may contribute to earlier improvements in symptom burden and cognitive function in individuals with Long COVID. Further studies are warranted.
Link (Frontiers in Neuroscience) [Provisionally accepted, currently only abstract]
Suzanne D Vernon, Candace Rond, Jennifer Bell, Brindisi Butler, Sara Isolampi, Annaleah Otteson, Pearl Phalwane, Samantha Mower, Shad Roundy, David Kaufman, Alan Brian Cash, Lucinda Bateman
Background
Long COVID is characterized by fatigue, cognitive dysfunction, and other persistent symptoms. This randomized, double-blind, controlled trial evaluated the efficacy of oral oxaloacetate (OAA) in improving fatigue and cognitive function in adults with Long COVID.
Methods
Sixty-nine participants were randomized to receive either 2,000 mg/day of OAA or control for 42 days. Primary outcome was fatigue reduction measured by the Chalder Fatigue Questionnaire (CFQ). Secondary and exploratory outcomes included the DePaul Symptom Questionnaire Short Form (DSQ-SF), health-related quality of life (RAND-36), cognitive function (DANA Brain Vital), and time upright (UP Time).
Results
No significant difference in CFQ fatigue reduction was observed between groups.
However, the OAA group showed significantly greater improvements in DSQ-SF fatigue and total symptom burden 21 days into the trial. Cognitive performance improved significantly in the OAA group, with strong correlations between symptom response and cognitive gains. OAA was well tolerated.
Conclusions
OAA may contribute to earlier improvements in symptom burden and cognitive function in individuals with Long COVID. Further studies are warranted.
Link (Frontiers in Neuroscience) [Provisionally accepted, currently only abstract]
Last edited: