RESTORE ME: A RCT of Oxaloacetate for Improving Fatigue in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)
Alan B. Cash, Suzanne D. Vernon, Candace Rond, Saeed Abbaszadeh, Jen Bell, Brayden Yellman, David Kaufman
*[Provisionally accepted, full text not yet available]*
Abstract
The energy metabolite oxaloacetate is significantly lower in the blood plasma of ME/CFS subjects. A previous open-label trial with oxaloacetate supplementation significantly reduced ME/CFS fatigue.
In a follow-on trial, 82 ME/CFS subjects were enrolled in a 3-month randomized double blinded controlled trial using 2,000 mg oxaloacetate or control/day. The primary endpoints were safety and a reduction in fatigue from baseline. Secondary and exploratory endpoints reviewed functional capacity, and general health status.
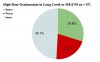
Results: Anhydrous enol-oxaloacetate (oxaloacetate) was well tolerated at the doses tested. Oxaloacetate significantly lowered fatigue from baseline by >25%, whereas the control group was not significant at ~10% reduction. Intergroup analysis of oxaloacetate and control measured shifted fatigue to lower levels in the oxaloacetate group (P= 0.0039), but with no significant shift in the control group. The oxaloacetate group had a higher percentage of subjects achieve a > 25% reduction in fatigue compared to the control group (P< 0.05). A subset of subjects that comprised 40.5% of the oxaloacetate group were "Enhanced Responders" with a 63% average fatigue reduction.
Both physical and mental fatigue were improved by oxaloacetate. Oxaloacetate is well-tolerated and helps to reduce fatigue in ME/CFS.
Link (Frontiers in Neurology)
Alan B. Cash, Suzanne D. Vernon, Candace Rond, Saeed Abbaszadeh, Jen Bell, Brayden Yellman, David Kaufman
*[Provisionally accepted, full text not yet available]*
Abstract
The energy metabolite oxaloacetate is significantly lower in the blood plasma of ME/CFS subjects. A previous open-label trial with oxaloacetate supplementation significantly reduced ME/CFS fatigue.
In a follow-on trial, 82 ME/CFS subjects were enrolled in a 3-month randomized double blinded controlled trial using 2,000 mg oxaloacetate or control/day. The primary endpoints were safety and a reduction in fatigue from baseline. Secondary and exploratory endpoints reviewed functional capacity, and general health status.
Results: Anhydrous enol-oxaloacetate (oxaloacetate) was well tolerated at the doses tested. Oxaloacetate significantly lowered fatigue from baseline by >25%, whereas the control group was not significant at ~10% reduction. Intergroup analysis of oxaloacetate and control measured shifted fatigue to lower levels in the oxaloacetate group (P= 0.0039), but with no significant shift in the control group. The oxaloacetate group had a higher percentage of subjects achieve a > 25% reduction in fatigue compared to the control group (P< 0.05). A subset of subjects that comprised 40.5% of the oxaloacetate group were "Enhanced Responders" with a 63% average fatigue reduction.
Both physical and mental fatigue were improved by oxaloacetate. Oxaloacetate is well-tolerated and helps to reduce fatigue in ME/CFS.
Link (Frontiers in Neurology)