Chandelier
Senior Member (Voting Rights)
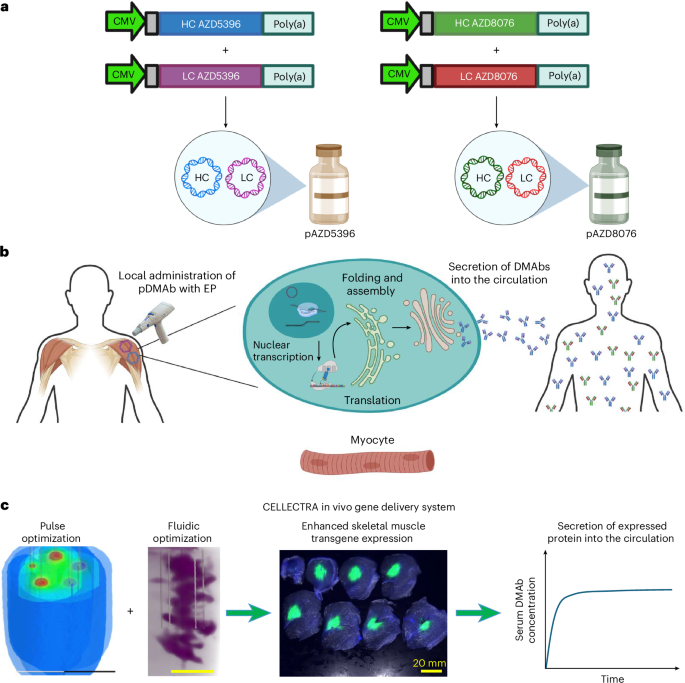
Safety and pharmacokinetics of SARS-CoV-2 DNA-encoded monoclonal antibodies in healthy adults: a phase 1 trial - Nature Medicine
In a phase 1 trial, intramuscular injection of synthetic plasmid DNA encoding monoclonal antibodies against SARS-CoV-2 was safe and well tolerated and did not elicit antidrug antibodies.
Pablo Tebas, Ami Patel, Joseph T. Agnes, Elizabeth M. Parzych, Amanda Baer, Maria Caturla, Sukanya Ghosh, Mansi Purwar, Nicole Bedanova, Chungdhak Tsang, Knashawn Morales, Dinah Amante, Paul D. Fisher, Joseph R. Francica, Laurent Humeau, Daniel W. Kulp, Jesper Pallesen, Paul Leon, Mark Esser, Trevor R. F. Smith & David B. Weiner
Abstract
Local intramuscular administration of synthetic plasmid DNA (pDNA) encoding monoclonal antibodies (mAb) offers an alternative to recombinant protein-based mAb delivery.In this phase 1 dose-escalation study, we evaluated the safety, tolerability and pharmacokinetics of a pDNA cocktail encoding AZD5396 and AZD8076, modified versions of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) neutralizing mAb cocktail tixagevimab/cilgavimab in healthy adults.
Participants received up to four intramuscular doses of pDNA encoding both DNA-based mAbs (DMAbs), administered using CELLECTRA electroporation.
The primary endpoints were safety and pharmacokinetics.
All 44 participants received at least one dose; DMAbs were detected in 100% of evaluable participants (n = 39), with serum concentrations reaching a peak of 1.61 µg ml−1.
Sustained expression was observed in all participants during the 72 weeks of follow-up.
The study product was well tolerated, with no product-related serious adverse events reported.
Exploratory analyses demonstrated binding to multiple SARS-CoV-2 Spike protein variants and neutralizing activity in a standard pseudovirus assay.
No antidrug antibodies were detected across approximately 1,000 serum samples using validated tiered assays.
To our knowledge, these data represent the first-in-human proof-of-concept that synthetic pDNA DMAb technology permits the durable in vivo production of a functional mAb cocktail.
This study further underscores the collective importance of synthetic design, formulation and delivery to achieve biologically relevant expression of gene-encoded biologics.
DMAb delivery may represent a long-acting, scalable, cold-chain-independent platform against a wide range of diseases that can be targeted with mAbs and their derivatives.
