Dolphin
Senior Member (Voting Rights)
https://www.tandfonline.com/doi/full/10.1080/21641846.2025.2455876
Research Article
Stellate Ganglion Block reduces symptoms of SARS-CoV-2-induced ME/CFS: A prospective cohort pilot study
Deborah L. Duricka
&
Luke D. Liu
Received 16 Nov 2024, Accepted 16 Jan 2025, Published online: 06 Feb 2025
ABSTRACT
Background
Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) is a debilitating condition characterized by fatigue, orthostatic intolerance (OI), post-exertional malaise (PEM) and unrefreshing sleep. Our previous work has shown that modulating the autonomic nervous system can alleviate symptoms of Long COVID, which shares striking similarities with ME/CFS.
Objective
Determine the effect of stellate ganglion block (SGB) on symptoms of ME/CFS.
Methods
Subjects who met the WHO criteria for Long COVID and the Institute of Medicine criteria for ME/CFS were treated with sequential bilateral SGBs separated by 18–24 hours for three consecutive weeks (n = 10). At baseline, and at 2-weeks and 2-months post-treatment, we collected subjective assessments (SF-36 and DSQ2) of symptoms, objective assessments of orthostatic intolerance and cognitive performance, and saliva to measure morning cortisol. During the entire study period, a wearable device collected physiological data several nights a week to measure sleep parameters.
Results
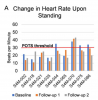
DSQ2 measures of PEM, Unrefreshing Sleep, Cognitive Impairment, and OI improved significantly following treatment. SF-36 measures of Vitality, Physical Function, and Social Function improved significantly following treatment. Objective symptoms of POTS associated with infectious onset resolved following treatment. Objective measures of cognitive impairment were reduced following treatment, most notably in the areas of Immediate and Delayed Recognition. Morning cortisol and measures of sleep architecture did not change significantly following treatment.
Conclusions
Symptoms of ME/CFS were reduced after treatment with SGBs in this small prospective cohort pilot study. Given the lack of FDA-approved treatments for ME/CFS, replication of results in a large clinical trial is warranted.
Research Article
Stellate Ganglion Block reduces symptoms of SARS-CoV-2-induced ME/CFS: A prospective cohort pilot study
Deborah L. Duricka
&
Luke D. Liu
Received 16 Nov 2024, Accepted 16 Jan 2025, Published online: 06 Feb 2025
ABSTRACT
Background
Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) is a debilitating condition characterized by fatigue, orthostatic intolerance (OI), post-exertional malaise (PEM) and unrefreshing sleep. Our previous work has shown that modulating the autonomic nervous system can alleviate symptoms of Long COVID, which shares striking similarities with ME/CFS.
Objective
Determine the effect of stellate ganglion block (SGB) on symptoms of ME/CFS.
Methods
Subjects who met the WHO criteria for Long COVID and the Institute of Medicine criteria for ME/CFS were treated with sequential bilateral SGBs separated by 18–24 hours for three consecutive weeks (n = 10). At baseline, and at 2-weeks and 2-months post-treatment, we collected subjective assessments (SF-36 and DSQ2) of symptoms, objective assessments of orthostatic intolerance and cognitive performance, and saliva to measure morning cortisol. During the entire study period, a wearable device collected physiological data several nights a week to measure sleep parameters.
Results
DSQ2 measures of PEM, Unrefreshing Sleep, Cognitive Impairment, and OI improved significantly following treatment. SF-36 measures of Vitality, Physical Function, and Social Function improved significantly following treatment. Objective symptoms of POTS associated with infectious onset resolved following treatment. Objective measures of cognitive impairment were reduced following treatment, most notably in the areas of Immediate and Delayed Recognition. Morning cortisol and measures of sleep architecture did not change significantly following treatment.
Conclusions
Symptoms of ME/CFS were reduced after treatment with SGBs in this small prospective cohort pilot study. Given the lack of FDA-approved treatments for ME/CFS, replication of results in a large clinical trial is warranted.