S4ME interview with Dr Sadie Whittaker.
A: welcome to Dr. Sadie Whittaker thank you for joining us for this chat. Over on the Science for ME forums we've collected a number of questions and we're gonna run through those and just talk to you about the work that Solve are doing. Broadly speaking on many categories,quite a lot on the research side.
So the first thing that we were looking to talk to you about was your biobank registry and app. Efforts that you’ve made to set those up and put those in place. Really the question at the moment is with those three, where are you at, how are they coming along and when do you see them going fully active?
SW: So first thank you for having me, I'm happy to be speaking to you today and I appreciate you taking the time to speak to us about our work. The biobank and registry is I think , if I have to pick one thing that we were doing in research this would be it. I come from an oncology background and spent most of my career in the biopharma industry in clinical development and really saw how big data it's a buzzword but how big data set transformed what we knew about oncology.
Sobreast cancer used to be a single disease and it's now, it’s tens of different diseases you know because of the genetic profiling in the information that we were able to get from big data.
I really think that's a real dynamic. I think it's absolutely critical and so
I see the biobank and registry as incredibly important and so we've been
building that and designing it in collaboration with patients, physicians and the community so that we can create a registry and biobank that is tailored for the end user.
We're asking people to give a bunch of their own personal information and so we want to make sure that
1) it's the information that they feel important and
2) that it's going to be useful for them too.
It's not just data that’s entered into a computer and that's the end of it.
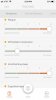
So some of the things we have been doing, a big part is we've been creating a symptom tracking app. And that was really based on a passion that I have that seeing information at single time point. It's information right, but it's so unlimited. It's the difference between the photograph and a moving picture. So right, the information we get from looking at photographs versus watching a movie and so I felt passionately that we wanted to do longitudinal data capture.
So we went out the community and said ‘what would be like the top five symptoms we'd want to capture?’, ‘how would you want to capture them?’, ‘how would you want to see data from those by symptoms?’, ‘how often would you want to capture them?’ and really worked with members of the community through that survey as well as from phone interviews to understand how to develop and design an app.
So we're really pleased where that’s got to right now.
That's really sort of been the hold up of rolling out the registry.
It’s that we really wanted components central to the whole design.
So that's fairly far along. We're working with the developers and we're hoping to take it and serve into testing probably over the next few weeks.
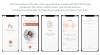
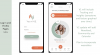
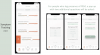
So we're getting really close, and we'd be happy to share some a sneak peek with your community to see some early screenshots of the app in terms of what it looks like. So we'll share those with you and you can push them out. But yeah, that's kind of where we're at with the app.
And then the other thing that I'm really excited about with the registry is we've been forging a partnership with the Cure ME group in the UK.
They already have a great repository and so could we work with them so that we could merge those datasets? And maybe they could use the
app too and so that we start to build a global depository not these disparate pieces of information. So with them we finally found the ? and they've been great partners and we're hopefully going to be able to launch that as part of it. And then once we've got them on board, my goal is to reach out to other groups in Australia, or you know, around the world, just come with your data. And so that we can start to see trends and ??
A: that's fantastic. One of the questions that our members had was
‘Would the registry, and or, the app be usable by those who are in the States?’
SW: yep
A: So obviously, from what you've just said, the intention is very much
that the international community is able to get involved?
SW: Yeah I want to make it as easy as possible.
We're casting a fairly wide net in terms of allowing people to enter their data. We are going to capture whether they being clinician confirmed some of diagnostic criteria kind of on the back end so that we can pass out the information.
But given how difficult it is for many people to get to get a diagnosis from a clinician we felt that it was important to be wide, right, and that as long as we could pass it out on the back end we wanted to encourage people to enter their data if they felt like they were really suffering with this disease.
So both in terms of that, but then gets your point but also globally. So we'll have the partnership with the UK group and then the registry that we're setting up will be available to everyone around the world.
A: Brilliant, and with regard to the registry will that be a source of potential recruits for trials is that a potential use for it?
SW: Yeah definitely. So how the empirical case use of it [will work]
is that we'll have this incredibly rich, both in terms of data points and in
terms of number of people, incredibly rich data sources ?? selection.
And so, as a researcher, we're going to create this interface where you can go in and search for ‘I want patients of a certain age who had the disease for a certain amount of time’ and it will spit out that information, that data from those patients. So you as a researcher will be able to access that.
And then you can request the specimens, and so it doesn't matter if those specimens are in the UK or the US or in Australia. Wherever they are they'll be tied to the information on the database, and so they can get shipped from wherever they are.
So that's kind of the empirical use of it.
Your point about ‘will it be a way to connect patients to or the research that's ongoing’, absolutely.
So patients, probably when they consent initially, will consent to be contacted if the research opportunity presents itself and researchers have that option to say to us ‘we're looking for patients, in this age group with this kind of disease etc’ and we can connect the two.
A: Brilliant. The main reason for asking that is that, to be honest truly, before this interview it occurred to me with our efforts with the NICE guideline reviews here in the UK. Really one of the goals would be to remove graded exercise therapy and the PACE style CBT from being viewed as treatments for ME, which obviously could have quite an impact on what services are actually offered for patients within the NHS. And at the moment, any researchers here in the UK would go to the chronic fatigue syndrome clinics principally to try and find patients for them. Now if NICE remove from the guidelines graded exercise therapy and CBT then there is a question about do the clinics still continue to exist or not? So I saw the registry as a potential answer if, should that ever happen.
It's much more, you know, a way for, particularly uk-based in that example, uk-based researchers to find potential recruits for their studies.
SW: Yeah, no absolutely, that’s going to be possible through the registry, so the other thing I'll tell you about it, I'm quite excited about this idea too.
So when you as a researcher, when you search the data it's going to automatically tell you if another researcher has used those specimens
and this data in another study. And it’ll show you what they did, what
their results were and give you the opportunity to connect them so in that
way we hope that the research will just continue to build and build and
build.
So everybody's not going to it fresh. The potential is to look at how certain datasets and biosamples have been looked at previously.
A: Brilliant, brilliant. We do have a question about patient involvement and how important you see that but I think what you've just explained about the registry and you know how intrinsically involved patients will need to be with that really answers how seriously you view that as it as important.
SW: Yeah a little bit, to me it's absolutely fundamental.
So, you know, patients are partners in everything that we do and so you've heard already how we've integrated them into the designing of the registry and that app. We will also integrate them into a kind of Advisory Council who sit above the registry and when people apply to use the data from the registry they'll apply to this council. And we'll have patients integrated into that so they can see what kind of questions are coming in. So that's one thing.
A second thing is anybody who's part of the registry gets to suggest their own research. So we're going to democratize the research questions and people are going to be able to submit what they believe are interesting questions and people will be able to up vote them. And based on which ones are up voted we will push them to researchers and see if we can get get them interested in researching specific areas.
So that's another one.
And then the third one is I want to encourage the patient community to interrogate the data themselves. Right I'm sure among the data of the patient community there are statisticians and epidemiologists.
We want it so anybody who uses the registry can apply to use the data so we want to encourage the [players]?? not always with the traditional
researchers to be able to delve into the data. So those some areas on the on the registry specifically on the Ramsay, and I can give you another example.
So again we have a group of people reviewers who help us decide which proposals to fund. And for the first time last year we incorporated six patient reviewers onto that body of people so that they can weigh in and decide whether or not this was valuable research.
A: fantastic so the patient involvement particularly in a field which has, it’s possibly fair to say, had a patient exclusion for so long, you know,
obviously makes a huge difference to the patient population when they see their voice has been taken seriously, and are included in the ongoing process.
SW: yeah that whole ?? concept that I'm sure you've seen, right you have the amount you spend in clinical care is this teeny tiny bit of the iceberg that you share with the doctor and then the rest of the iceberg underwater. It’s all of the information that you have as an individual living with the disease and so I think it's ridiculous to think that you would not partner with someone who had all of that information to try and better understand what's going on.