alicec
Senior Member (Voting Rights)
I wasn’t sure where to put this. It is general background information about metabolism, but since it is directly relevant to energy production in PWME and hence fatigue, I put it here.
Recently I needed to refresh my memory about how some amino acids are broken down for energy.
I thought I would make a summary of this since several recent studies have shown that defects in other energy production pathways mean that PWME may increasingly rely on amino acids as an energy source - see for example the Fluge and Mella and Armstrong studies.
So for people who like to know how things work, the summary shows the different places that amino acids feed in to energy pathways and explains why different amino acids have different metabolic effects.
First a few basic concepts.
Normally around 10% of our energy comes from breakdown of amino acids. The vast majority comes from breakdown of glucose (derived from carbohydrates) and fatty acids (derived predominantly from triglycerides).
At the heart of energy production is the Kreb’s cycle, AKA the Citric acid cycle (CAC), AKA as the Tricarboxyllic acid (TCA) cycle (citrate is a tricarboxylic acid, ie it contains three carboxyl groups - COOH).
Glucose and fatty acids are broken down to feed into the cycle at a single (different) entry point while amino acids are broken down to substances with multiple entry points.
This diagram from Wikipedia shows the multiple points for amino acid feed-in.
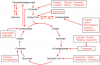
Note that glucose feeds in at pyruvate, after undergoing a multi-step process known as glycolysis.
Fatty acids feed in at acetylCoA after undergoing a multi-step process known as beta oxidation.
Note also that two related pathways are illustrated which help to explain the different metabolic effects of some amino acids.
These additional pathways are gluconeogenesis and ketogenesis.
Gluconeogenesis, as the name implies, is the synthesis of glucose from non-carbohydrate carbon sources. In the diagram it is shown by the arrow from pyruvate to oxaloacetate and then to phosphoenolpyruvate. From this point, the glucose synthetic pathway is glycolysis in reverse.
This is an energy expensive pathway that is invoked when glucose supplies, which are absolutely required by certain cell types, become limiting (such as during starvation, fasting, vigorous exercise or low carbohydrate intake).
The initiating enzyme is pyruvate carboxylase, which converts pyruvate to oxaloacetate.
There are additional substrates for gluconeogenesis which are not shown in the diagram. These include:-
i) lactate and alanine which are sent from exercising muscles to the liver for conversion to pyruvate and then to glucose (look up the Cori cycle and glucose-alanine cycle to understand this better) ;
ii) glutamine which in the kidney and small intestine is converted to alpha ketoglutarate and hence via the Kreb’s cycle to oxaloacetate and eventually glucose
iii) the glycerol backbone of triglycerides, which in the liver feeds into the reverse glycolysis pathway.
Ketogenesis, as the name implies, is the production of ketones from fatty acids and some amino acids. The starting point is acetylCoA which is converted in the liver to acetoacetate and then the other ketone bodies, viz beta hydroxybutyrate and acetone. It is shown in the diagram as a reversible loop from acetylCoA.
Ketogenesis is invoked when cellular conditions limit the production of Kreb’s cycle intermediates such as oxaloacetate and acetylCoA accumulates. Unavailability of glucose is the common trigger or excess breakdown of fatty acids.
The ketones can be sent from the liver to cells in need of energy; there the ketones are converted back to acetylCoA and used in the Kreb’s cycle.
So now we can see that amino acids can be categorised in three ways depending on their metabolic effects, ie whether they end up as acetylCoA and hence feed ketogenesis, or whether they end up as pyruvate or Kreb’s cycle intermediates and so feed gluconeogenesis. Some do both.
Ketogenic: Leu, Lys
Ketogenic and Glucogenic: Phe, Trp, Tyr, Ile
Glucogenic: Ala, Arg, Asp, Asn, Cys, Glu, Gln, His, Met, Pro, Ser, Val, Thr
Note that in some animals Thr is also ketogenic, but not in humans.
If you want to know more detail of the individual amino acid catabolic pathways, illustrations can be found here and here; the latter site also has a detailed discussion of gluconeogenesis, ketogenesis and the control thereof.
Fluge and Mella, in their seminal paper characterising the energy defects in ME/CFS, further subdivided the glucogenic category into those which were converted to pyruvate (their category 1) and those which replenished intermediates in the Kreb’s cycle – the anapleurotic amino acids (their category 3).
Their category 2 amino acids were those with ketogenic potential (regardless of whether they were also glucogenic).
They did this because it fitted the pattern of amino acid usage in the women with ME/CFS who they studied. Blood levels of category 2 and 3 amino acids were reduced, indicating increased usage, while category 1 amino acids were unaffected.
They concluded that this pattern of usage indicated a problem with pyruvate to acetylCoA conversion; in face of this difficulty, amino acids which didn’t feed into this step were increasingly used to compensate.
They then showed that the pyruvate dehydrogenase complex (PDH), which catalyses the pyruvate to acetylCoA conversion, is inhibited in ME/CFS.
This was a result of upregulation of genes coding for several enzymes which inhibit PDH, viz SIRT4, members of the PDH kinase family of enzymes (PDK) and the transcription factor PPRD.
What is driving this upregulation is not yet known.
One small practical spin-off of these studies is that it might be worthwhile considering supplementation of some or all of the amino acids which are not broken down to pyruvate as a means of boosting energy production.
There was some discussion of this strategy in another place with mixed success reported.
I have tried it, also with mixed success. It definitely helps with energy but you may need to experiment with the amino acid mixture. I became a little depressed if I continued too long with an essential amino acid mixture. Pulsing is better.
I interpreted this to mean some interference with neurotransmitter balance. This in turn might reflect too much leucine interfering with uptake of neurotransmitter precursors such as phenylalanine and tyrosine, since they used the same transporter (the large, neutral amino acid transporter).
ETA Tryptophan, another important neurotransmitter precursor, uses this transporter also.
Recently I needed to refresh my memory about how some amino acids are broken down for energy.
I thought I would make a summary of this since several recent studies have shown that defects in other energy production pathways mean that PWME may increasingly rely on amino acids as an energy source - see for example the Fluge and Mella and Armstrong studies.
So for people who like to know how things work, the summary shows the different places that amino acids feed in to energy pathways and explains why different amino acids have different metabolic effects.
First a few basic concepts.
Normally around 10% of our energy comes from breakdown of amino acids. The vast majority comes from breakdown of glucose (derived from carbohydrates) and fatty acids (derived predominantly from triglycerides).
At the heart of energy production is the Kreb’s cycle, AKA the Citric acid cycle (CAC), AKA as the Tricarboxyllic acid (TCA) cycle (citrate is a tricarboxylic acid, ie it contains three carboxyl groups - COOH).
Glucose and fatty acids are broken down to feed into the cycle at a single (different) entry point while amino acids are broken down to substances with multiple entry points.
This diagram from Wikipedia shows the multiple points for amino acid feed-in.

Note that glucose feeds in at pyruvate, after undergoing a multi-step process known as glycolysis.
Fatty acids feed in at acetylCoA after undergoing a multi-step process known as beta oxidation.
Note also that two related pathways are illustrated which help to explain the different metabolic effects of some amino acids.
These additional pathways are gluconeogenesis and ketogenesis.
Gluconeogenesis, as the name implies, is the synthesis of glucose from non-carbohydrate carbon sources. In the diagram it is shown by the arrow from pyruvate to oxaloacetate and then to phosphoenolpyruvate. From this point, the glucose synthetic pathway is glycolysis in reverse.
This is an energy expensive pathway that is invoked when glucose supplies, which are absolutely required by certain cell types, become limiting (such as during starvation, fasting, vigorous exercise or low carbohydrate intake).
The initiating enzyme is pyruvate carboxylase, which converts pyruvate to oxaloacetate.
There are additional substrates for gluconeogenesis which are not shown in the diagram. These include:-
i) lactate and alanine which are sent from exercising muscles to the liver for conversion to pyruvate and then to glucose (look up the Cori cycle and glucose-alanine cycle to understand this better) ;
ii) glutamine which in the kidney and small intestine is converted to alpha ketoglutarate and hence via the Kreb’s cycle to oxaloacetate and eventually glucose
iii) the glycerol backbone of triglycerides, which in the liver feeds into the reverse glycolysis pathway.
Ketogenesis, as the name implies, is the production of ketones from fatty acids and some amino acids. The starting point is acetylCoA which is converted in the liver to acetoacetate and then the other ketone bodies, viz beta hydroxybutyrate and acetone. It is shown in the diagram as a reversible loop from acetylCoA.
Ketogenesis is invoked when cellular conditions limit the production of Kreb’s cycle intermediates such as oxaloacetate and acetylCoA accumulates. Unavailability of glucose is the common trigger or excess breakdown of fatty acids.
The ketones can be sent from the liver to cells in need of energy; there the ketones are converted back to acetylCoA and used in the Kreb’s cycle.
So now we can see that amino acids can be categorised in three ways depending on their metabolic effects, ie whether they end up as acetylCoA and hence feed ketogenesis, or whether they end up as pyruvate or Kreb’s cycle intermediates and so feed gluconeogenesis. Some do both.
Ketogenic: Leu, Lys
Ketogenic and Glucogenic: Phe, Trp, Tyr, Ile
Glucogenic: Ala, Arg, Asp, Asn, Cys, Glu, Gln, His, Met, Pro, Ser, Val, Thr
Note that in some animals Thr is also ketogenic, but not in humans.
If you want to know more detail of the individual amino acid catabolic pathways, illustrations can be found here and here; the latter site also has a detailed discussion of gluconeogenesis, ketogenesis and the control thereof.
Fluge and Mella, in their seminal paper characterising the energy defects in ME/CFS, further subdivided the glucogenic category into those which were converted to pyruvate (their category 1) and those which replenished intermediates in the Kreb’s cycle – the anapleurotic amino acids (their category 3).
Their category 2 amino acids were those with ketogenic potential (regardless of whether they were also glucogenic).
They did this because it fitted the pattern of amino acid usage in the women with ME/CFS who they studied. Blood levels of category 2 and 3 amino acids were reduced, indicating increased usage, while category 1 amino acids were unaffected.
They concluded that this pattern of usage indicated a problem with pyruvate to acetylCoA conversion; in face of this difficulty, amino acids which didn’t feed into this step were increasingly used to compensate.
They then showed that the pyruvate dehydrogenase complex (PDH), which catalyses the pyruvate to acetylCoA conversion, is inhibited in ME/CFS.
This was a result of upregulation of genes coding for several enzymes which inhibit PDH, viz SIRT4, members of the PDH kinase family of enzymes (PDK) and the transcription factor PPRD.
What is driving this upregulation is not yet known.
One small practical spin-off of these studies is that it might be worthwhile considering supplementation of some or all of the amino acids which are not broken down to pyruvate as a means of boosting energy production.
There was some discussion of this strategy in another place with mixed success reported.
I have tried it, also with mixed success. It definitely helps with energy but you may need to experiment with the amino acid mixture. I became a little depressed if I continued too long with an essential amino acid mixture. Pulsing is better.
I interpreted this to mean some interference with neurotransmitter balance. This in turn might reflect too much leucine interfering with uptake of neurotransmitter precursors such as phenylalanine and tyrosine, since they used the same transporter (the large, neutral amino acid transporter).
ETA Tryptophan, another important neurotransmitter precursor, uses this transporter also.
Last edited:
