InitialConditions
Senior Member (Voting Rights)
Editor’s Summary
Epstein-Barr virus (EBV) has been making waves as a candidate driver of diseases like multiple sclerosis and Long Covid. It has also long been linked to systemic lupus erythematosus (SLE), although the “why” behind this link has not been defined. Here, Younis et al. provide evidence for a link between EBV infection and disease development. The authors found, using a new strategy to identify EBV-infected cells by RNA sequencing, that infected B cells were transcriptionally distinct from their uninfected counterparts. EBV-infected B cells exhibited features associated with antigen presentation, and this programming seemed likely to be directly driven by the EBV protein EBNA2. These EBV-infected B cells with antigen-presenting abilities had the capacity to activate autoreactive helper T cells, setting off a chain reaction where those T cells could activate other autoreactive B cells, including uninfected ones. In vitro studies in B cell lines provided functional support for this hypothesis. These data suggest that EBV infects and reprograms autoreactive B cells that, in turn, drive the systemic autoimmune response in SLE.Abstract
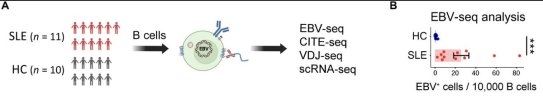
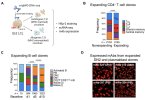
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease characterized by antinuclear antibodies (ANAs). Epstein-Barr virus (EBV) infection has been epidemiologically associated with SLE, yet its role in pathogenesis remains incompletely defined. Here, we developed an EBV-specific single-cell RNA-sequencing platform and used it to demonstrate that EBV infection reprograms autoreactive antinuclear antigen B cells to drive autoimmunity in SLE. We demonstrated that, in SLE, EBV+ B cells are predominantly CD27+CD21low memory B cells that are present at increased frequencies and express ZEB2, TBX21 (T-bet), and antigen-presenting cell transcriptional pathways. Integrative analysis of chromatin immunoprecipitation sequencing (ChIP-seq), assay for transposase-accessible chromatin sequencing (ATAC-seq), and RNA polymerase II occupancy data revealed EBV nuclear antigen 2 (EBNA2) binding at the transcriptional start sites and regulatory regions of CD27, ZEB2, and TBX21, as well as the antigen-presenting cell genes demonstrated to be up-regulated in SLE EBV+ B cells. We expressed recombinant antibodies from SLE EBV+ B cells and demonstrated that they bind prototypical SLE nuclear autoantigens, whereas those from healthy individuals do not. We further found that SLE EBV+ B cells can serve as antigen-presenting cells to drive activation of T peripheral helper cells with concomitant activation of related EBV− antinuclear double-negative 2 B cells and plasmablasts. Our results provide a mechanistic basis for EBV being a driver of SLE through infecting and reprogramming nuclear antigen-reactive B cells to become activated antigen-presenting cells with the potential to promote systemic disease–driving autoimmune responses.This is currently paywalled:
Last edited: