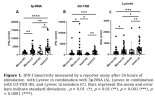
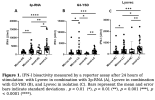
Exaggerated IFN-I Response in Long COVID PBMCs Following Exposure to Viral Mimics
Purpose
Long COVID (LC) is a long-term debilitating disease of which the exact pathophysiology is unknown. A dysregulated immune response resulting in hyperresponsive immune cells is hypothesized as a key mechanism in the development of LC. Several studies suggest that acute infections can leave lasting epigenetic changes, which result in heightened immune reactivity. Upon stimulation, these primed immune cells may exhibit exaggerated responses. This form of epigenetic memory can contribute to altered immune dynamics, particularly in response to induction of type I Interferons (IFN-I) pathway activation using a viral mimic. Therefore, we investigated if LC patients exhibit a hyperresponsive response towards viral mimics in comparison with healthy controls (HC).
Methods
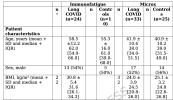
PBMCs of two distinct LC cohorts, characterized by a different disease course and duration, were collected and transfected using Lyovec with the cGAS and RIG-I agonists G3-YSD and 3p-RNA followed by measurement of IFN-I bioactivity with a reporter cell line.
Results
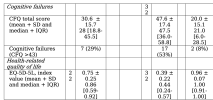
Transfection of PBMCs of LC patients with the cGAS and RIG-I agonists resulted in increased IFN-I bioactivity in comparison with HC. Unsupervised hierarchical clustering revealed two distinct clusters, each predominantly composed of either patients or HC. In addition, a moderate correlation between RIG-I stimulation with 3p-RNA and fatigue severity scores was found.
Conclusion
These data show a hyperresponsive phenotype of immune cells of LC patients upon stimulation with viral mimics. The current availability of biologicals and small molecule inhibitors that interfere with aberrant IFN-I pathway activation underscores the importance of pursuing future investigations into this phenomenon.
Web | DOI | PDF | Journal of Clinical Immunology | Open Access
Humer, Bart; Berentschot, Julia C.; van Helden-Meeuwsen, Cornelia G.; Bek, L. Martine; de Bie, Maaike; Defesche, Tobias M.; Boly, Chantal A.; Drost, Manon; Hellemons, Merel E.; Dik, Willem A.; Versnel, Marjan A.
Purpose
Long COVID (LC) is a long-term debilitating disease of which the exact pathophysiology is unknown. A dysregulated immune response resulting in hyperresponsive immune cells is hypothesized as a key mechanism in the development of LC. Several studies suggest that acute infections can leave lasting epigenetic changes, which result in heightened immune reactivity. Upon stimulation, these primed immune cells may exhibit exaggerated responses. This form of epigenetic memory can contribute to altered immune dynamics, particularly in response to induction of type I Interferons (IFN-I) pathway activation using a viral mimic. Therefore, we investigated if LC patients exhibit a hyperresponsive response towards viral mimics in comparison with healthy controls (HC).
Methods
PBMCs of two distinct LC cohorts, characterized by a different disease course and duration, were collected and transfected using Lyovec with the cGAS and RIG-I agonists G3-YSD and 3p-RNA followed by measurement of IFN-I bioactivity with a reporter cell line.
Results
Transfection of PBMCs of LC patients with the cGAS and RIG-I agonists resulted in increased IFN-I bioactivity in comparison with HC. Unsupervised hierarchical clustering revealed two distinct clusters, each predominantly composed of either patients or HC. In addition, a moderate correlation between RIG-I stimulation with 3p-RNA and fatigue severity scores was found.
Conclusion
These data show a hyperresponsive phenotype of immune cells of LC patients upon stimulation with viral mimics. The current availability of biologicals and small molecule inhibitors that interfere with aberrant IFN-I pathway activation underscores the importance of pursuing future investigations into this phenomenon.
Web | DOI | PDF | Journal of Clinical Immunology | Open Access