Simon M
Senior Member (Voting Rights)
In simple terms, the headline one is the strongest of several candidates for each genetic signal (though more than one gene covered by a genetic signal may play a part in the illness.Dumb question time: what’s the difference between a candidate gene, like the ones on this list, and the 8 ‘headline’ genes, in very simple terms? Thanks
This is from the DecodeME blog:
>
DecodeME identifies top genes
DecodeME has started searching for the treasure, and has identified likely genes using several methods. These methods looked at genes within the genetic signals – the peaks on the Manhattan plot.
The most powerful method searched those genes to find ones whose activity levels (the amount of protein they make) are known to go up or down depending on what form a particular variant within the ME/CFS genetic signal takes. This means that the activity of those genes is related to someone having ME/CFS – a strong clue that those genes might be a cause of ME/CFS.
<
The second paragraph talks about a method called eQTL, which I will try to explain here in a bit more detail.
We start with the genetic signal for ME/CFS. Basically, candidate genes are nearby, but which are playing a part in causing ME?
One way of finding out is to see if gene activity changes in people with ME.
Lets take a genetic signal for ME/CFS that has a particular known variant We want to know if the gene behaves differently in peoople who have that variant. If there is no difference, the gene probably isn't doing anything relevant.
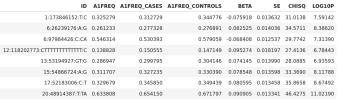
There is a large public database (GTEX) that has such data: it shows gene expression of each in people with different variants. So, the method is to find the variant associated with ME, then look to see if nearby genes show different levels of activity in people with that variant.
Here is an example (not from the study) for the gene PLG, in this case showing its mRNA expression level in the brain's frontal cortex. The known variant has either the letter C or the letter T. We have two copies of each set of DNA (one from each parent), so each person has a 'genotype' of CC, CT or TT.
As you can see, the gene is less active (X axis) where people have CC and more when they are TT, with CT somewhere in between.

Note that we don't know the DNA sequence of the gene itself - just its activity level and how that changes with the genotype.
And it does NOT show that the variant causes gene expression itself. More likely it is another, unseen, variant affecting both the gene and ME status.
Back to DecodeME. If gene activity correlates with ME status (through the variant) then it is an interesting gene. I think 43 genes were identified this way, and 29 were classified as priority genes (presumably, taking into account other factors as well).
ADDED
Here is Fig 4 showing which genesare associated with ME/CFS.

Fig. 4: Approximate Bayes factor posterior probability (PPH4) that mRNA expression and ME/CFS traits are associated and share a single causal variant.
Thirty-four protein coding genes (y-axis) with at least one expression QTL (eQTL; GTEx-v10) within a FUMA-defined ME/CFS interval that show colocalisation with the eQTLs for at least one of 49 GTEx-v10 tissues (xaxis).
Green circles indicate that increased ME/CFS risk allele is associated with increased gene expression for eQTLs in the FUMA-defined
interval. Blue circles indicate that increased ME/CFS risk allele is associated with decreased gene expression for eQTLs in the FUMA-defined
interval, and vice-versa. The area of each circle indicates the test’s value of PPH4 (posterior probability for single shared causal variant): smaller circles indicate 0 < PPH4 < 0.75, and larger ones indicate PPH4 ≥ 0.75.
Last edited: