Robert 1973
Senior Member (Voting Rights)
These comments are from the media coverage thread but I’m posting here as they are relevant to the study design and interpretation of data:
Please correct me if I’m misunderstanding.
Being covered on Sky news now. Only the Psychologist on the panel was asked to comment. She stated that what people are pointing out is that many of the participants in the study had 'self reported diagnosis'. Am I correct in recalling that only those with a medically confirmed diagnosis were invited to provide samples, or am I confused? I do remember that I had to wait to see if I would be called to provide a sample.
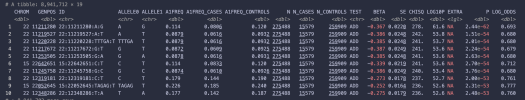
Given that the study has found genetic associations, does the fact that the study used self-reported symptoms not strengthen the result? I can see that it was an unavoidable weakness in the study design, which could have been used as an explanation if it had found no associations but, to my limited understanding, finding these associations despite the unavoidable weaknesses in the design, suggests that these signals could be stronger and more numerous if there was a way to screen patients more thoroughly (eg examined and tested by physicians as part of the study).There were two parts to this. The study was of people who reported they had a diagnosis from a health professional. The study screening questionnaire also asked people about their symptoms. Only people who met the criteria for either IOM or Canadian consensus definitions were invited to provide DNA samples. I'm not sure, but I think about 85% of people who signed up saying they had a diagnosis from a health professional also met the criteria.
DecodeME included a question on postexertional malaise that went beyond asking about simple exertion intolerance stressing the need for an extending period of symptom flare. Though, as this is a media thread, I don't want to start an extended debate about that here.
But I want to clarify this isn't self diagnosis, it's not even simply self report of a medical diagnosis. It won't be perfect, but I think it will be pretty good.
Please correct me if I’m misunderstanding.
Last edited: