ME/CFS Prevalence: Affects ~1% of the general population in the United States, with women being affected at a rate 3 times higher than men.
ME/CFS Impact: Causes significant disability worldwide, impacting patients, family members, and society through healthcare costs and loss of productivity.
ME/CFS Research Challenges: Lack of specific diagnostic tests and targeted therapies limit effective management, and the growing number of long COVID cases highlights the urgency of advancing research.
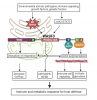
ME/CFS Complexity: ME/CFS is a complex condition influenced by cellular dysfunction, genetic predisposition, and environmental factors, leading to diverse and unpredictable symptoms.
Mitochondrial Dysfunction: Mitochondrial dysfunction, potentially contributing to fatigue and post-exertional malaise, is a key area of research in understanding ME/CFS.
Therapeutic Potential: Understanding mitochondrial dysfunction in ME/CFS may lead to new therapeutic targets and treatments.
Mitochondrial Dysfunction in ME/CFS: Mitochondrial dysfunction is a potential contributor to the symptoms of ME/CFS, including fatigue, cognitive impairment, and immune system abnormalities.
Mechanism of Mitochondrial Dysfunction: The exact mechanism of mitochondrial dysfunction in ME/CFS is unclear, but it may be a cause or effect of the underlying pathogenesis.
Importance of Mitochondrial Function: Mitochondria play a crucial role in cellular energy production and overall cellular homeostasis, and their dysfunction can contribute to various diseases.
Mitochondrial Dysfunction in ME/CFS: Patients with ME/CFS exhibit mitochondrial dysfunction, characterised by reduced ATP synthesis, increased glycolytic activity, and decreased oxygen extraction during exercise.
Metabolic Profiling in ME/CFS: Metabolic profiling of blood samples from ME/CFS patients reveals specific differences compared to healthy subjects, suggesting potential metabolic perturbations.
Brain MRS Findings in ME/CFS: Brain MRS studies have shown elevated lactate levels in ME/CFS patients, potentially linked to neuroinflammation and mitochondrial dysfunction.
Mitochondrial Dysfunction in ME/CFS: Despite abnormalities in mitochondrial function, ME/CFS is not classified as a mitochondrial disease due to the lack of specific genetic changes.
Mitochondrial DNA and ME/CFS: Studies have not found significant associations between mitochondrial DNA variations and ME/CFS, but certain mtDNA haplogroups and SNPs are linked to specific symptoms.
Oxidative Stress in ME/CFS: ME/CFS patients exhibit increased oxidative stress, characterised by elevated levels of oxidative markers and reduced antioxidant levels.
CoQ10 Function: CoQ10 is an antioxidant that protects cells from oxidative stress and is involved in ATP production.
Mitochondrial Dysfunction: Mitochondrial dysfunction can lead to increased ROS production, potentially contributing to oxidative stress and cellular dysfunction in ME/CFS.
Peroxisomal Dysfunction: Peroxisomal dysfunction, suggested by plasma metabolomic profiling in ME/CFS patients, may further contribute to oxidative stress and cellular dysfunction.
WASF3 Overexpression: Overexpression of WASF3 protein is linked to decreased mitochondrial respiration and disrupted mitochondrial respiratory supercomplexes.
Mechanism of Action: WASF3 interacts with CIII and destabilises CIV subunits, leading to mitochondrial dysfunction.
ME/CFS Association: Elevated WASF3 levels and ER stress markers are observed in skeletal muscle biopsies of ME/CFS patients.
ER Stress and Mitochondrial Dysfunction: ER stress, potentially triggered by viral infections, disrupts mitochondrial function and contributes to oxidative stress.
WASF3 as a Potential Mediator: Increased WASF3 levels, observed in ME/CFS, could be part of an immune response to perceived stimuli, potentially linking ER stress to mitochondrial dysfunction.
Impact on ME/CFS Symptoms: Impaired mitochondrial function in ME/CFS may explain exercise intolerance and slow recovery from fatigue due to ATP depletion, slow oxidative metabolism, and increased oxidative stress.
Mitochondrial Dysfunction Impact: Extends beyond energy insufficiency, potentially affecting brain function and contributing to neurocognitive symptoms.
Immune System Dysregulation: Altered immunoglobulin, cytokine, and cellular component profiles, along with evidence of chronic inflammation and gut microbiome changes, suggest immune system dysregulation.
Mitochondrial Role in Immune Cells: Mitochondria are crucial for immune cell function, particularly T cells, which undergo metabolic reprogramming to respond to pathogens.
WASF3 Function: Regulates actin cytoskeleton, essential for T cell and B cell receptor signalling, and potentially involved in immune metabolism and function.
WASF3 Structure: Contains a N-terminal WHD and a C-terminal VCA region, which binds actin and Arp2/3 for regulating actin polymerization.
WASF3 Metabolic Impact: May reprogram cell metabolism by suppressing mitochondrial oxidative phosphorylation and enhancing glycolysis.
WASF3 and Neuroinflammation: WASF3, highly expressed in the brain, may be involved in neuroinflammation, a key feature of ME/CFS.
WASF3 and Immune Response: Elevated WASF3 levels activate p38 MAPK, a key cellular stress mediator, potentially as a feedback signal to promote mitochondrial biogenesis.
WASF3 and Long COVID: Investigating the role of WASF3 in immune cells could provide insights into the chronic inflammatory characteristics observed in Long COVID patients.
Fatigue Syndromes and Viral Infections: COVID-19, like ME/CFS, often follows an infection, with EBV being a common trigger for ME/CFS.
Long COVID and ME/CFS Symptoms: Up to 20% of COVID-19 patients experience lingering fatigue, cognitive impairment, and other symptoms resembling ME/CFS.
Mitochondrial Dysfunction in Fatigue: Emerging research suggests mitochondrial dysfunction as a common factor in both ME/CFS and long COVID, potentially contributing to fatigue and exercise intolerance.
Mitochondrial Dysfunction Treatment: Therapeutic approaches to correct mitochondrial dysfunction could provide symptomatic relief in ME/CFS patients.
Treatment Approaches: Mitochondrial biogenesis enhancers, metabolic modulators, and antioxidant supplements have been explored to enhance mitochondrial function and improve symptoms in ME/CFS patients.
Cofactor Supplementation: Coenzyme Q10 (CoQ10) and NADH, essential cofactors for mitochondrial function, have been tested as supplements to improve symptoms in ME/CFS patients.
Mitochondrial Dysfunction in ME/CFS: Mitochondrial dysfunction is a key factor in ME/CFS, with studies exploring treatments targeting mitochondrial function.
Treatment Trials: Various treatments, including D-Ribose, KPAX002, sodium dichloroacetate (DCA), red ginseng (HRG80), and quercetin, have shown some benefits in improving symptoms.
Limitations and Future Directions: Despite some positive results, these treatments have not significantly impacted ME/CFS management due to modest benefits and variable efficacy.
Mitochondrial Dysfunction: Recurring theme in ME/CFS pathophysiology, potentially contributing to multi-system symptoms.
Clinical Heterogeneity: Challenges in diagnosis and treatment due to varying symptoms and lack of specific biomarkers.
Therapeutic Potential: Salubrinal, a PP1 inhibitor, shows promise in reducing ER stress and restoring mitochondrial function, but challenges remain.
WASF3 as a Potential Mediator: Recent study suggests WASF3 might be involved in mitochondrial dysfunction in ME/CFS, linking to cellular energy, ER stress, and immunity.
Oxidative Stress and ER Stress: Oxidative stress and redox imbalance can trigger ER stress, and its role in ME/CFS, particularly in relation to WASF3, is an area for further investigation.
Targeting WASF3 for Insights: Targeting WASF3 through ER stress modulation could provide insights into the molecular pathways of ME/CFS pathogenesis and serve as a potential biomarker for treatment efficacy.
WASF3's Role in Metabolism: WASF3 may regulate metabolism by disrupting mitochondrial respiration and promoting glycolysis.
WASF3's Role in Immunity: WASF3 may support immune system activation by promoting metabolic shifts.
Impact on ME/CFS: Prolonged activation of the WASF3 pathway may contribute to chronic inflammation and energy deficiency in ME/CFS.