I was surprised to get an email saying that this was out prior to final publication (which is delayed only due to administrative delays caused by the festive season)... I don't recall approving something like this (I don't like pre-prints, I feel it muddies the waters when there are two versions of a paper in circulation, and can cause mistakes to become spread through the broader community) but anyway... enough of that.
Context for this study:
- IMO the most useful property of these cell lines is that we already have them and we can grow them in huge quantities, reasonably cheaply, compared with the labour and reagents costs involved in sampling patients, so this makes them good for screening for "barn door" changes that are worth validating in primary cells.
- So, this study was a relatively cheap and quick screen (smallish sample) to see whether anything clearly stood out as metabolically wrong so that we can have some pilot data to help us fund next steps that are less likely to be dead ends.
Main finding:
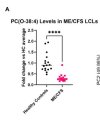
The big, clear thing that stood out is that the ME/CFS cell lines have way more lipid content that I don't think is explicable by culture medium (discussed in paper, basically the content of this stuff in the medium is really low).
I've attached a simple graphical representation of this for people's ease of viewing (every circle is one lipid, and every lipid above the central dotted line is above healthy control levels).
We can speculate endlessly about why it's happening and what the consequences might be, and specifically in a B cell context... if you're interested in all of the plausible speculation that I could come up with, I would direct people to the Discussion... it's too much and too dense to repeat here.
I think the most obvious and potentially relevant consequence would be differences in cell membrane fluidity and lipid raft dynamics (due to the specific classes of compounds involved, including cholesterol). This would affect BCR engagement. A next step would be to verify whether this lipid profile change occurs in primary B cell populations (or other cell populations of interest) and whether it is associated with aberrant BCR engagement. That would be a very detailed and probably expensive study, so it's not been started yet.
Sorry if the "next step" wasn't clear to anyone, I originally had a far less vague final sentence of abstract and Conclusions to make this clearer, but reviewers were asking for very expensive and lengthy validation experiments clearly out of scope so I confined speculation and future steps to the Discussion to emphasise that this was speculation about next steps and not direct conclusions of the data. @Jonathan Edwards I dislike vague interpretation as much as the next guy.
Replies to posts:
Any reason to think you would be able to see these high lipids in blood serum, high triglycerides?
One elephant in the room is whether what we've seen reflects a body-wide metabolic change or a cell type specific one. Gun to my head, I'd say this is chiefly evidence for the latter, because metabolic control in different lymphoid cell populations is tied up in complex signalling processes that aren't present elsewhere and which can cause total 180-degree flips in which fuel sources are being used. This is also why we need to validate these observations in primary cells next... complexities introduced by the EBV immortalisation used to create LCLs could explain these results. The fact that it's such a group-specific effect means that there is at least some sort of signalling aberration in the parental B cells used to make the cell lines, if not a metabolic one. So we'll see what the primary cells tell us. I think there is
clearly something different about the original B cells, it just may not be the exact same differences that we're seeing in the cell lines.
We are also looking at other tissues and we've seen
preliminary evidence of enlarged lipid droplets in fibroblasts which suggests something similar, but I don't want to run away with that just yet.
Noting since lipids seem like something that would be changed with BMI:
Sadly we did this experiment years ago, with older cohorts which we received from a clinic that has now closed down, so some of the older experiments like this one have limited clinical information attached to them. What we do have, as a strength, is confident CCC ascertainment. I am including more detailed characterisation plus FUNCAP in our newer work but this will take time to become apparent in the publication pipeline.
I will say, though; the circulating cells used to make the cell lines will have been in culture prior to immortalisation, and the cell lines have been cultured through enough passages that metabolically they shouldn't really "remember" what environment they were in effectively "weeks" prior. So while BMI is a possible confounding factor I don't suspect it would be in this case. I also don't suspect this particular group had many or even any pwME who were obese (obesity being a factor that may associate with elevated circulating lipid levels which could contribute to cellular hyperlipidaemia). What we are measuring here is pretty far removed from blood. It would have to result from a pretty stable epigenetic (outside of the regions altered by EBV) or regulatory effect.
Any reason to think you would be able to see these high lipids in blood serum, high triglycerides?
I'm sure this has been speculated to death in the various blood-based metabolism studies that have reported related findings... I will refrain here from personally commenting on this as this paper only puts forward evidence for things happening inside cells and I don't want to go beyond that. Until we see more mature evidence in our other tissue samples I will be keeping the focus to first B cells and then other circulating immune cell populations.
Hoping someone will explain the implications for those of us at the back...
Basically, it suggests something wrong with B cells that relates to their metabolism, so we need to next go to fresh cells from people's blood to validate what we saw here. If something similar does exist in cells from people with the illness it could arise from many potential reasons but the most obvious consequence would be abnormal immune function due to differences in an important protein complex called the B Cell Receptor.
This looks like a lot of work and some interesting results.
I don't have the knowledge to understand all the details but I have read parts of it and found the discussion readable and refreshingly clear about the limitations and what needs to be done next.
I was also pleased to see the name ME/CFS being used throughout and the authors not wasting time trying to explain what ME/CFS is. I like the straightforward getting on with the science.
Thank you for your work @DMissa and @MelbME.
Do you have funding for pursuing some of the next steps you indicate?
Thank you, Trish, while the experiment was pretty quick I think I've spent something like 3 years analysing this data and trying to write it up as carefully as I could!!! I hope it is written clearly and within the evidence. I tried really hard to achieve this.
TLDR: immortalised B cells from pwME clearly have more lipid content, especially molecules that would lead to more rigid cell membranes. Next, this all needs to be verified in fresh, unimmortalised cells from better-characterised patients and in relation to relevant potential issues such as BCR engagement.