Dolphin
Senior Member (Voting Rights)
Preliminary abstract, see post #13 for final paper.
-----------------
https://www.sciencedirect.com/science/article/abs/pii/S0960896624003353
571P Muscular metabolic plasticity in 3D in vitro models against systemic stress factors in ME/CFS and long COVID-19
Author links
1
Institute For Bioengineering Of Catalonia BIST, Barcelona, Spain
2
ICREA Institució Catalana de Recerca i Estudis Avançats, Barcelona, Spain
3
Institut d'Investigacions Biomèdiques August Pi i Sunyer, Barcelona, Spain
4
Universitat de Barcelona, Barcelona, Spain
Available online 4 October 2024, Version of Record 4 October 2024.
https://doi.org/10.1016/j.nmd.2024.07.171Get rights and content
Myalgic encephalomyelities/ chronic fatigue syndrome and long COVID-19 are clinically challenging, multi-symptomatic conditions with multiple overlapping symptoms.
Unfortunately, contemporary research is directly being done on patients which risks exacerbating their symptoms.
Using our 3-D in vitro skeletal muscle tissues we have mapped the progression of functional, physiological, and metabolic adaptations of the tissues in response to patient sera over time.
During short exposure we treated the tissues for 48 hours with patient sera.
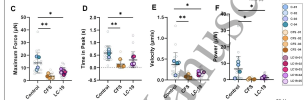
The contractile profiles of these tissues were severely compromised.
Transcriptomic analyses of these short exposure samples showed an absence of significant differentially expressed genes between ME/CFS and LC-19.
The analyses revealed an upregulation of glycolytic enzymes especially of PDK4, suggesting a switch away from Oxidative Phosphorylation as well as a decline in DRP1, involved in mitochondrial fission.
Subsequent structural analyses confirmed hypertrophy in myotubes and hyperfused mitochondrial networks.
Mitochondrial oxygen consumption capacity, evaluated through the MitoStress test, was also elevated, as was the non-mitochondrial respiration confirming the shift to glycolysis.
Interestingly, at short exposures of 48 hours, the muscle tissues appeared to be adapting to the stress factors by upregulating glycolysis and increasing the muscular metabolic volume.
Prolonging the exposure to 96 and 144 hours induced high fatiguability, and fragility in tissues.
The mitochondria, at longer exposures, appeared to be fragmented and assumed a toroidal conformation indicating a change in mitochondrial membrane potential.
We hypothesize that the disease progresses through an intermediary stress-induced hypermetabolic state, ultimately leading to severe deterioration of muscle function.
This is the first account of research that proposes acquired metabolic plasticity in 3D skeletal muscles exposed to ME/CFS and Long COVID-19 sera.
S. Mughal, F. Andújar-Sánchez, M. Sabater-Arcis, J. Fernández-Costa, J. Ramón-Azcón,
571P Muscular metabolic plasticity in 3D in vitro models against systemic stress factors in ME/CFS and long COVID-19,
Neuromuscular Disorders,
Volume 43, Supplement 1,
2024,
104441.162,
-----------------
https://www.sciencedirect.com/science/article/abs/pii/S0960896624003353
571P Muscular metabolic plasticity in 3D in vitro models against systemic stress factors in ME/CFS and long COVID-19
Author links
1
Institute For Bioengineering Of Catalonia BIST, Barcelona, Spain
2
ICREA Institució Catalana de Recerca i Estudis Avançats, Barcelona, Spain
3
Institut d'Investigacions Biomèdiques August Pi i Sunyer, Barcelona, Spain
4
Universitat de Barcelona, Barcelona, Spain
Available online 4 October 2024, Version of Record 4 October 2024.
https://doi.org/10.1016/j.nmd.2024.07.171Get rights and content
Myalgic encephalomyelities/ chronic fatigue syndrome and long COVID-19 are clinically challenging, multi-symptomatic conditions with multiple overlapping symptoms.
Unfortunately, contemporary research is directly being done on patients which risks exacerbating their symptoms.
Using our 3-D in vitro skeletal muscle tissues we have mapped the progression of functional, physiological, and metabolic adaptations of the tissues in response to patient sera over time.
During short exposure we treated the tissues for 48 hours with patient sera.
The contractile profiles of these tissues were severely compromised.
Transcriptomic analyses of these short exposure samples showed an absence of significant differentially expressed genes between ME/CFS and LC-19.
The analyses revealed an upregulation of glycolytic enzymes especially of PDK4, suggesting a switch away from Oxidative Phosphorylation as well as a decline in DRP1, involved in mitochondrial fission.
Subsequent structural analyses confirmed hypertrophy in myotubes and hyperfused mitochondrial networks.
Mitochondrial oxygen consumption capacity, evaluated through the MitoStress test, was also elevated, as was the non-mitochondrial respiration confirming the shift to glycolysis.
Interestingly, at short exposures of 48 hours, the muscle tissues appeared to be adapting to the stress factors by upregulating glycolysis and increasing the muscular metabolic volume.
Prolonging the exposure to 96 and 144 hours induced high fatiguability, and fragility in tissues.
The mitochondria, at longer exposures, appeared to be fragmented and assumed a toroidal conformation indicating a change in mitochondrial membrane potential.
We hypothesize that the disease progresses through an intermediary stress-induced hypermetabolic state, ultimately leading to severe deterioration of muscle function.
This is the first account of research that proposes acquired metabolic plasticity in 3D skeletal muscles exposed to ME/CFS and Long COVID-19 sera.
S. Mughal, F. Andújar-Sánchez, M. Sabater-Arcis, J. Fernández-Costa, J. Ramón-Azcón,
571P Muscular metabolic plasticity in 3D in vitro models against systemic stress factors in ME/CFS and long COVID-19,
Neuromuscular Disorders,
Volume 43, Supplement 1,
2024,
104441.162,
Last edited by a moderator: