Nitric oxide signalling in cardiovascular health and disease
Charlotte Farah, Lauriane Y. M. Michel, Jean-Luc Balligand
Abstract
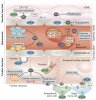
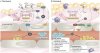
Nitric oxide (NO) signalling has pleiotropic roles in biology and a crucial function in cardiovascular homeostasis. Tremendous knowledge has been accumulated on the mechanisms of the nitric oxide synthase (NOS)–NO pathway, but how this highly reactive, free radical gas signals to specific targets for precise regulation of cardiovascular function remains the focus of much intense research.
In this Review, we summarize the updated paradigms on NOS regulation, NO interaction with reactive oxidant species in specific subcellular compartments, and downstream effects of NO in target cardiovascular tissues, while emphasizing the latest developments of molecular tools and biomarkers to modulate and monitor NO production and bioavailability.
Key Points
PubMed | Link (paywalled)
Charlotte Farah, Lauriane Y. M. Michel, Jean-Luc Balligand
Abstract
Nitric oxide (NO) signalling has pleiotropic roles in biology and a crucial function in cardiovascular homeostasis. Tremendous knowledge has been accumulated on the mechanisms of the nitric oxide synthase (NOS)–NO pathway, but how this highly reactive, free radical gas signals to specific targets for precise regulation of cardiovascular function remains the focus of much intense research.
In this Review, we summarize the updated paradigms on NOS regulation, NO interaction with reactive oxidant species in specific subcellular compartments, and downstream effects of NO in target cardiovascular tissues, while emphasizing the latest developments of molecular tools and biomarkers to modulate and monitor NO production and bioavailability.
Key Points
- The three isoforms of nitric oxide synthase subserve distinct, but coordinated, functions through their subcellular confinement in cardiac and vascular cells
- The redox environment dictates the fate of nitric oxide and pathophysiological effects
- Multiple regulatory points of downstream effectors ensure signalling specificity and allow therapeutic modulation
- New techniques for monitoring nitric oxide bioavailability will allow efficient tailoring of treatment
PubMed | Link (paywalled)