Introduction: A recent study reported a favorable effect of vitamin B12 injections/oral folic acid support in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) patients. Recently, vitamin B12 nasal drops were developed as an alternative to the vitamin B12 injections. As no data are available on efficacy of this formulation, we studied vitamin B12 serum levels, the physical activity scale of the RAND-36, the number of steps on an activity meter, and the fatigue and concentration scales of the CIS20r questionnaires, before and after 3 months of treatment in ME/CFS patients.
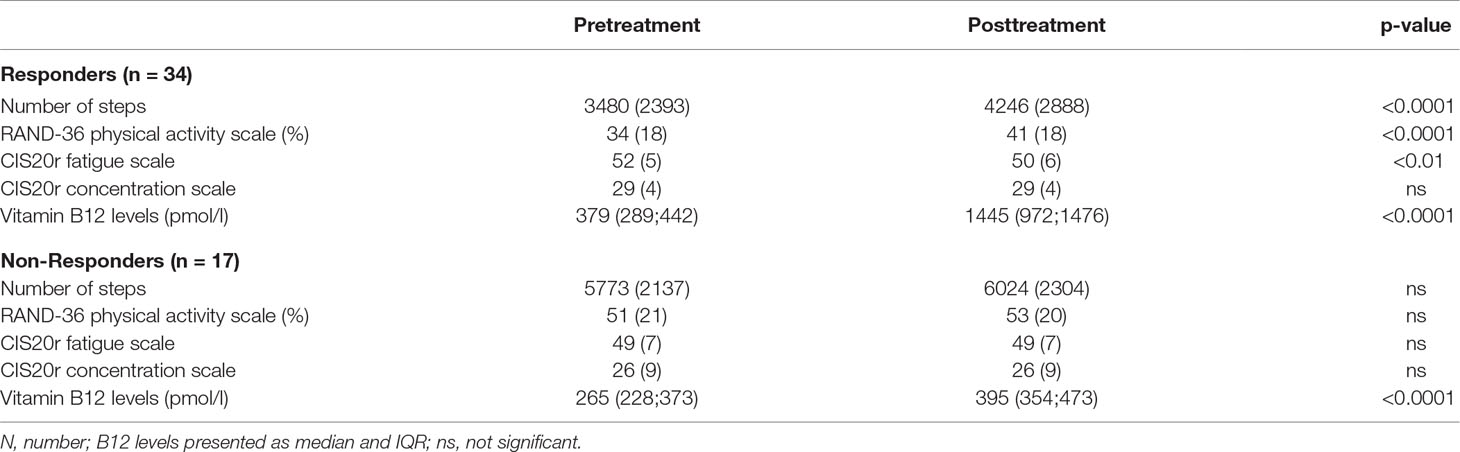
Methods and Results: Fifty-one patients completed all measurements. Forty-four were female. Mean age was 42 years, and mean disease duration was 16 years. Median vitamin B12 levels before treatment were 328 (244–429) pmol/l, and 973 (476–1,476) pmol/l after treatment. Thirty-four patients reported a favorable response to treatment. In the non-responders, only a small but significant increase in vitamin B12 levels was observed. In contrast, in responders, the number of steps, the physical activity scale of the RAND-36, and the vitamin B12 serum levels increased significantly. The CIS20r fatigue scale decreased significantly, and the CIS20r concentration scale was unchanged.
Conclusions: Nasal drop vitamin B12 administration resulted in a significant increase in vitamin B12 serum levels and therefore may be effective. This pilot study suggest that the nasal drops may be used as an alternative to injections because two thirds of ME/CFS patients reported a positive effect, accompanied by an increased number of steps, improvement of the RAND-36 physical functioning scale and the CIS20r fatigue scale, and a significant increase in serum vitamin B12 levels.