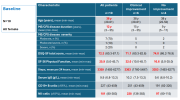
Self-reported questionnaires
Patients completed self-reported questionnaires every 2 weeks through the 3-months run-in period and until week 16 after start of treatment. Questionnaires were then administered every 4 weeks until week 40 for patients who had four treatments, and until week 60 for those who received maintenance injections, and at extended follow-up at 66 and 92 weeks. Questionnaires included the Norwegian-language versions of the Short Form-36 questionnaire for health-related quality of life (SF-36, ver. 1.2) (
42), and the DePaul Symptom Questionnaire - Short Form (DSQ-SF) for ME/CFS symptoms (
43). The Norwegian translation of DSQ-SF is based on the translation of the complete DePaul Symptom Questionnaire. Patients also recorded their perceived Function level in per cent according to a table with examples (
Supplementary protocol).
Fitbit activity armbands
Patients used a Fitbit Charge 5 activity armband through follow-up from week −12 to 40, continuously recording steps per 24 h and resting heart rate. A Data Protection Impact Assessment was performed prior to study start. To protect the participants’ privacy, we used pseudonymization toward third parties. Each participant Fitbit account was set up using a study-specific e-mail address, initials instead of name and a fictitious date of birth. Fitbit’s terms of use complied with the General Data Protection Regulation (GDPR) directive. Fitbit activity data from each participant were downloaded at the study centre regularly, using the Fitbit web API. For each participant we registered an Oauth 2.0 application with type set as “personal.” The scopes were set to heartrate + sleep + activity.