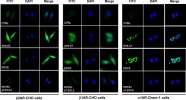
We used receptor‐transfected cells to demonstrate specific autoantibody binding to the receptors. Sera from a POTS subject who demonstrated activating autoantibodies to β1AR, β2AR, and α1AR, sera from a healthy control subject and rabbit polyclonal antibodies to each receptor as positive controls were separately incubated with these cells without and with an excess of their respective ECL2 target peptide. These images are shown in
Figure 6. There was significant binding to each of the receptors from the POTS sera, which was completely blocked by preabsorption with the specific ECL2 peptides. No significant binding to the cells from the control sera was observed.