I don't see any individual level data for illness duration in the study. Just a mean and range in Table 1. And I don't see anything about NK relationship to duration. Maybe it's from a talk?I'm I'm not misremembering I think the NK cell findings here were somewhat related to illness duration (I'm not actually sure if anyone checked whether the correlation between response and NK count was stronger than response and illness duration), which would make that quite possible (people with long-term illnesses "responding" better for whatever reasons).
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Trial Report Plasma cell targeting with the anti-CD38 antibody daratumumab in ME/CFS -a clinical pilot study, 2025, Fluge et al
Possible, but probably more likely that I might be misremembering. I guess the table does at least suggest the possibility for a relationship.I don't see any individual level data for illness duration in the study. Just a mean and range in Table 1. And I don't see anything about NK relationship to duration. Maybe it's from a talk?
ryanc97
Senior Member (Voting Rights)
Whose theory would be?
OK, it has been the received wisdom in generic immunology circles for decades, but it has nothing much to recommend it.
I think you will find forum members are up to speed on the other stuff!!
Theory from Olav Mella in his video or presentation that was recorded at Charite MECFS conference 2025.
See 14:28.
If it were a common theory, this is the first ever attempt at targeting the source of AABs, which I find odd.
Last edited:
ryanc97
Senior Member (Voting Rights)
That's what Im trying to figure out. Is it a one-shot impulse style thing where during the CFS trigger, B cells went rogue?"Furthermore, IgG4 production by antibody-secreting cells can be markedly shorter lived than for other IgG subclasses, requiring continuous input from newly differentiating B cells. Indeed, rituximab (anti-CD20) therapy for B cell depletion has been shown to be particularly beneficial in autoimmune diseases characterized by pathogenic IgG4 (auto)antibodies" The unique properties of IgG4 and its roles in health and disease.
And when rituximab was used in ME, it didn't help, even though SLPC was affected by the drug. And as you said, "Rituximab should work if the source is SLPC in theory." In reality it didn't work. Maybe the target wasn't correct, or they didn't use the drug in the optimal way in the study.
I think IgG4 makes sense because it's a blocking antibody and it's known to be noninflammatory and does not activate complement or ADCC, and there are no signs of inflammation or autoimmune tissue damage in ME. It can bind to some of the many proteins that we don't recognize yet as a target. for example, like in MuSK myastenia gravis.
The other question is why the disease hasn’t come back after stopping datratumumab if dara isn’t touching B cells?
Why new autoreactive plasma cells weren’t made
Also, from the charts response was clearly seen at T=6 weeks. ChatGPT says IGG has a half life of 3W. Once you stop the flow of toxic antibodies, 50% gets cleared out/consumed by 3 weeks. So at 6W you might be looking at 75% of toxic antibodies cleared out and the body responds
Last edited:
ryanc97
Senior Member (Voting Rights)
Well there is another thing that complicates things, the higher IGG4 count at baseline in responders vs non responders:
NK cell counts at t=0 - higher (238 vs 97 in 10^6/L)
IGG4 counts at t=0 - higher (0.6 vs 0.21 in g/L)
I know in this thread IGG4 has been discussed already. And JE said CD20 drugs like Ritux respond better to IGG4 things according to medical theory.
We know these things:
1. The non responders were 2 severe 2 moderate, while responders were 6 moderate. So non responders were worse off at baseline.
2. Non responders had lower IGG4 levels, which would imply that IGG4 being high is less correlated to symptom severity.
3. IGG4 is produced farther back in the B cell pipeline by SLPCs and differentiating B cells, while IGG4 is less produced by LLPCs.
4. Ritux helps with IGG4 problems, obviously because the pipeline further back is more CD20 than CD38.
5. From P3 RCT we know ritux has less effect. Since if IGG4 reduction was key, Ritux would have more effect.
Why would higher IGG4 result in less severity? Makes no sense to me.
Also for the 7 dose group, 3 were responders (patients 07, 08, 10) and 1 was not (09). Annoyingly, they gave the extra 3 doses to the 3 patients with the highest NK cells....
NK cell counts at t=0 - higher (238 vs 97 in 10^6/L)
IGG4 counts at t=0 - higher (0.6 vs 0.21 in g/L)
I know in this thread IGG4 has been discussed already. And JE said CD20 drugs like Ritux respond better to IGG4 things according to medical theory.
We know these things:
1. The non responders were 2 severe 2 moderate, while responders were 6 moderate. So non responders were worse off at baseline.
2. Non responders had lower IGG4 levels, which would imply that IGG4 being high is less correlated to symptom severity.
3. IGG4 is produced farther back in the B cell pipeline by SLPCs and differentiating B cells, while IGG4 is less produced by LLPCs.
4. Ritux helps with IGG4 problems, obviously because the pipeline further back is more CD20 than CD38.
5. From P3 RCT we know ritux has less effect. Since if IGG4 reduction was key, Ritux would have more effect.
Why would higher IGG4 result in less severity? Makes no sense to me.
Also for the 7 dose group, 3 were responders (patients 07, 08, 10) and 1 was not (09). Annoyingly, they gave the extra 3 doses to the 3 patients with the highest NK cells....
Last edited:
V.R.T.
Senior Member (Voting Rights)
During the Charite conference, it was claimed that an American pharma company is planning a trial of CAR T in ME/CFS, and Fluge said in his opinion it was too soon, maybe in 5 years.Maybe there’s some kind of CD38 targeting CAR-NK or CAR-T cell therapy that can better target and deplete CD38 plasmablasts while sparing CD38high NK cells and retaining sufficient ADCC cytotoxicity. Would really help test their theory and see if better responses can be produced in the lower NK baseline non-responders.
That’s the problem with monoclonals like dara and ritux they don’t effectively deplete all their target in a subset of patients (in cancer and autoimmune diseases too) leading to inadequate clinical responses and researchers questioning their pathomechanism theories when in reality it’s just the drug isn’t good enough.
Jonathan Edwards
Senior Member (Voting Rights)
deplete CD38 plasmablasts
Why would you want to target plasmablasts? I don't see them as of any particular relevance - just a brief transition phase before plasma cell.
This is all well above my pay grade, but someone involved in complement research recently told me high IgG4 may be a compensatory mechanism in an effort to tamp down overactive complement.Why would higher IGG4 result in less severity? Makes no sense to me.
leokitten
Senior Member (Voting Rights)
I just miswrote that sorry was reading something on plasma cells and plasmablasts and mixed it upWhy would you want to target plasmablasts? I don't see them as of any particular relevance - just a brief transition phase before plasma cell.
Andy
Senior Member (Voting rights)
A Correction on Plasma cell targeting with the anti-CD38 antibody daratumumab in myalgic encephalomyelitis/chronic fatigue syndrome—A clinical pilot study
In the Introduction section, paragraph 7, the last sentence incorrectly stated:
However, although specific functional autoantibodies were elevated during acute COVID-19 infection (14), the number and magnitude of autoreactivities to the exoproteome were similar in individuals with or without LC (17), suggesting that the pattern of functional autoantibody responses, rather than single pathogenic autoantibodies, may be relevant.
The correct sentence should read:
However, although specific functional autoantibodies were elevated during acute COVID-19 infection (14), the number and magnitude of autoreactivities to the exoproteome were similar in individuals with or without ME/CFS (17), suggesting that the pattern of functional autoantibody responses, rather than single pathogenic autoantibodies, may be relevant.
The original version of this article has been updated.
Link
In the Introduction section, paragraph 7, the last sentence incorrectly stated:
However, although specific functional autoantibodies were elevated during acute COVID-19 infection (14), the number and magnitude of autoreactivities to the exoproteome were similar in individuals with or without LC (17), suggesting that the pattern of functional autoantibody responses, rather than single pathogenic autoantibodies, may be relevant.
The correct sentence should read:
However, although specific functional autoantibodies were elevated during acute COVID-19 infection (14), the number and magnitude of autoreactivities to the exoproteome were similar in individuals with or without ME/CFS (17), suggesting that the pattern of functional autoantibody responses, rather than single pathogenic autoantibodies, may be relevant.
The original version of this article has been updated.
Link
Jonathan Edwards
Senior Member (Voting Rights)
Michael Scoma comments:
Daratumumab and ME/CFS
Daratumumab, an anti-CD38 monoclonal antibody approved for myeloma, is being explored in ME/CFS due to CD38’s role in immune signaling and inflammation. T
Targeting CD38 may help reset immune dysregulation seen in subsets of ME/CFS patients.
Going back to this odd tweet.
We don't seem to have discussed the possibility that blocking CD38 on a whole load of other cells is what made people feel better.
CD38 is on plasma cells but it has more than one important function in modulating T cell and NK responses. Maybe by chance this is the first trial of a T cell soothing treatment in ME/CF`S and it works. Nothing to do with antibodies.
If that were the case perhaps Fluge and Mella have stumbled on a fast lane route to a rtional effective therapy. CD38 might not be the best target, although if it is then they are home and dry already.
Jonathan Edwards
Senior Member (Voting Rights)
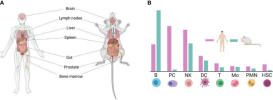
Shows expression of CD38 on various cell types. Plasma cells and NK cells have a lot.
Apparently there is very little on gamma delta T cells.
Last edited:
Utsikt
Senior Member (Voting Rights)
Jonathan Edwards
Senior Member (Voting Rights)
CD38 expression on cell types. Man and mouse.
Jonathan Edwards
Senior Member (Voting Rights)
at PC?
Plasma cell.
Utsikt
Senior Member (Voting Rights)
So you’re essentially proposing that B and NK cells might be red herring correlations, and that the effect (if it exists) of Dara is mediated by T cells?Going back to this odd tweet.
We don't seem to have discussed the possibility that blocking CD38 on a whole load of other cells is what made people feel better.
CD38 is on plasma cells but it has more than one important function in modulating T cell and NK responses. Maybe by chance this is the first trial of a T cell soothing treatment in ME/CF`S and it works. Nothing to do with antibodies.
If that were the case perhaps Fluge and Mella have stumbled on a fast lane route to a rtional effective therapy. CD38 might not be the best target, although if it is then they are home and dry already.
Are there any relationships between NK numbers and T cells that might explain why high NK cells might be a indicator of responding to Dara?
OrganicChilli
Senior Member (Voting Rights)
CD38 might not be the best target, although if it is then they are home and dry already.
Other than trialling different drugs, how would we know if there's a better target?