Mij
Senior Member (Voting Rights)
Abstract
Patients suffering from fibromyalgia often report stress and pain, with both often refractory to usual drug treatment. Magnesium supplementation seems to improve fibromyalgia symptoms, but the level of evidence is still poor. This study is a randomized, controlled, double-blind trial in fibromyalgia patients that compared once a day oral magnesium 100 mg (Chronomag®, magnesium chloride technology formula) to placebo, for 1 month.
The primary endpoint was the level of stress on the DASS-42 scale, and secondary endpoints were pain, sleep, quality of life, fatigue, catastrophism, social vulnerability, and magnesium blood concentrations. After 1 month of treatment, the DASS-42 score decreased in the magnesium and placebo groups but not significantly (21.8 ± 9.6 vs. 21.6 ± 10.8, respectively, p = 0.930). Magnesium supplementation significantly reduced the mild/moderate stress subgroup (DASS-42 stress score: 22.1 ± 2.8 to 12.3 ± 7.0 in magnesium vs. 21.9 ± 11.9 to 22.9 ± 11.9 in placebo, p = 0.003).
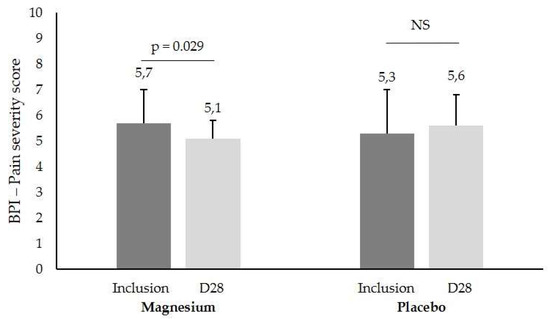
Pain severity diminished significantly (p = 0.029) with magnesium while the other parameters were not significantly different between both groups. These findings show, for the first time, that magnesium improves mild/moderate stress and reduces the pain experience in fibromyalgia patients. This suggests that daily magnesium could be a useful treatment to improve the burden of disease of fibromyalgia patients and calls for a larger clinical trial.
https://www.mdpi.com/2072-6643/14/10/2088#
Patients suffering from fibromyalgia often report stress and pain, with both often refractory to usual drug treatment. Magnesium supplementation seems to improve fibromyalgia symptoms, but the level of evidence is still poor. This study is a randomized, controlled, double-blind trial in fibromyalgia patients that compared once a day oral magnesium 100 mg (Chronomag®, magnesium chloride technology formula) to placebo, for 1 month.
The primary endpoint was the level of stress on the DASS-42 scale, and secondary endpoints were pain, sleep, quality of life, fatigue, catastrophism, social vulnerability, and magnesium blood concentrations. After 1 month of treatment, the DASS-42 score decreased in the magnesium and placebo groups but not significantly (21.8 ± 9.6 vs. 21.6 ± 10.8, respectively, p = 0.930). Magnesium supplementation significantly reduced the mild/moderate stress subgroup (DASS-42 stress score: 22.1 ± 2.8 to 12.3 ± 7.0 in magnesium vs. 21.9 ± 11.9 to 22.9 ± 11.9 in placebo, p = 0.003).
Pain severity diminished significantly (p = 0.029) with magnesium while the other parameters were not significantly different between both groups. These findings show, for the first time, that magnesium improves mild/moderate stress and reduces the pain experience in fibromyalgia patients. This suggests that daily magnesium could be a useful treatment to improve the burden of disease of fibromyalgia patients and calls for a larger clinical trial.
https://www.mdpi.com/2072-6643/14/10/2088#