Mij
Senior Member (Voting Rights)
Abstract
Post-acute sequelae of COVID (PASC) persist in many patients for weeks and months after recovery from initial SARS-CoV-2 infection. Recent evidence suggests that pathological changes in skeletal muscle may contribute significantly to ongoing pain and fatigue, particularly post-exertional malaise.
This study aimed to investigate the underlying mechanisms of PASC-related fatigue by examining skeletal muscle function and circulating factors in affected individuals.
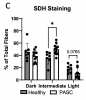
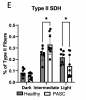
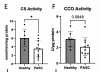
We conducted a cross-sectional case-control study of patients with fatigue-associated PASC who had experienced mild to moderate COVID-19 without hospitalization. Skeletal muscle biopsies revealed reduced mitochondrial respiration and content in PASC participants compared to healthy controls. This lower respiratory capacity was accompanied by markedly elevated circulating levels of soluble IL-2 receptor alpha subunit (sIL2R), a T cell-specific receptor.
In vitro experiments demonstrated that sIL2R directly impairs mitochondrial oxygen consumption and reduces mitochondrial complex III subunit protein levels in cultured muscle cells. These findings suggest a mechanism linking systemic immune dysregulation to muscle-specific mitochondrial dysfunction in PASC.
This work provides new insights into the pathophysiology of PASC identifying sIL2R as a promising therapeutic target for addressing mitochondrial deficits in PASC-related fatigue and opening avenues for developing targeted interventions.
LINK
Post-acute sequelae of COVID (PASC) persist in many patients for weeks and months after recovery from initial SARS-CoV-2 infection. Recent evidence suggests that pathological changes in skeletal muscle may contribute significantly to ongoing pain and fatigue, particularly post-exertional malaise.
This study aimed to investigate the underlying mechanisms of PASC-related fatigue by examining skeletal muscle function and circulating factors in affected individuals.
We conducted a cross-sectional case-control study of patients with fatigue-associated PASC who had experienced mild to moderate COVID-19 without hospitalization. Skeletal muscle biopsies revealed reduced mitochondrial respiration and content in PASC participants compared to healthy controls. This lower respiratory capacity was accompanied by markedly elevated circulating levels of soluble IL-2 receptor alpha subunit (sIL2R), a T cell-specific receptor.
In vitro experiments demonstrated that sIL2R directly impairs mitochondrial oxygen consumption and reduces mitochondrial complex III subunit protein levels in cultured muscle cells. These findings suggest a mechanism linking systemic immune dysregulation to muscle-specific mitochondrial dysfunction in PASC.
This work provides new insights into the pathophysiology of PASC identifying sIL2R as a promising therapeutic target for addressing mitochondrial deficits in PASC-related fatigue and opening avenues for developing targeted interventions.
LINK