My notes on the presentation from Dr Jarred Younger (@5h 27’):
A new,
simpler way to measure neuroinflammation
Younger presented findings from his study in which he used brain scans to reveal levels of metabolites and changes in temperature across the brain. He proposed that these measures are proxies for neuroinflammation, potentially a cause of ME/CFS. There were clear differences between patients and controls, with patients showing inflammation and increases in temperature in areas that could produce symptoms of fatigue and malaise. If validated and replicated, the new method would give a non-invasive approach that could prove valuable both in research and routine clinical diagnosis.
——
Younger's primary hypothesis is that ME/CFS is driven by neuroinflammation. The brain's main immune cells are called microglia. An infection in the body, such as influenza, leads to activation of microglia in the brain. The microglia produce proinflammatory cytokines, affecting nearby neurones, which leads to a “sickness response”. The sickness response, which includes fatigue, pain and the inability to concentrate, is designed to make us rest up so that the body can focus its resources on fighting off the infection.
Younger thinks that something goes wrong in ME/CFS so that following an initial infection, instead of microglia returning to their normal, "on patrol", state, they end up in a primed, hyperactive state. And in the primed state, minor triggers, perhaps exertion, lead microglia to produce cytokines all over again, making us feel sick. He says the specific ME/CFS symptoms are down to where in the brain microglia are concentrated, which can vary between patients.

Microglia. Top left: normal, patrolling microglia with extended arms; bottom right: activated “neuroinflammatory“ state. Top right: primed, hyperactive state.
Up until now, the only way to detect neuroinflammation (at least without autopsy) has been the PET method which involves injecting a radioactive tracer that lights up activated microglia on MRI scans. That was the method used by the Japanese PET MRI study a few years back; it is hideously expensive and unsafe to do repeatedly.
Younger used magnetic resonance spectroscopy (MRS), which is a good technique for identifying specific metabolites. Using some fancy physics, MRS can also reveal changes in brain temperature. Younger focused on the metabolite lactate which, as with exercise, indicates intense activity (or not enough oxygen to meet demand). He argued that this is a mark of neuroinflammation. He also identified an increase in temperature as another sign of neuroinflammation: such elevated temperatures “should only happen if you have a neuroinflammatory response, because you can’t think hard enough to heat up your brain.”
The results showed that both lactate and temperature increases differed markedly between patients and controls, with more lactate and higher temperatures in patients. Red areas are 1°F (0.6°C) higher than green areas.
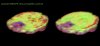
Specific areas of ME/CFS patients brains (left) show higher levels of lactate, in red, than for healthy controls (right).
Importantly, neuroinflammation occurred in areas of the brain, such as the amygdala and the insula, whose activation Younger said would account for ME/CFS symptoms.
Critically, said Younger, the increases in lactate and increases in temperature generally map together, giving him confidence that both were indeed measuring neuroinflammation.

Higher temperatures were recorded in ME/CFS patients’ brains than for controls
Younger noted that there was also a good match between the areas of neuroinflammation indicated by higher temperatures and high levels of lactate in his new study and the areas indicated by the original Japanese neuroinflammation study. This match is another reason for confidence in the method.
Overall, it makes for a strong story. However, as Younger himself pointed out, these are preliminary data (on 15 patients and 15 controls) that are yet to be published, replicated or validated.
Validation against the PET "gold standard" of neuroinflammation is critical. So what's needed is a study showing that, within individual patients, the areas of increased lactate and increased temperature map to microglial activation identified by the radioactive tracer. Younger said his group now have funds for this kind of work in fibromyalgia, but not ME/CFS.
However, Cort Johnson has just reported that Younger applied to the NIH for a grant for a bigger version of his current study, and the NIH asked him to add a PET study using radioactive tracer to validate the findings.
If everything works out, this new approach could prove to be very important, and not just in ME/CFS. No wonder that other researchers at the Stanford workshop were so interested in Younger’s work.
Note that Michael VanElzakker has pointed out that neuroinflammation is likely to be common in a number of diseases (though the patterns might well be different) — so neuroinflammation itself probably wouldn't be diagnostic.
——
Any chance of an opinion on this new work from
@Jonathan Edwards? Also
@Woolie?

