TiredMathematician
Established Member
Abstract
Myalgic Encephalomyelitis (ME), also known as chronic fatigue syndrome (CFS), is a debilitating and heterogeneous disorder marked by persistent fatigue, post-exertional malaise, cognitive impairment, and multisystem dysfunction. Despite its prevalence and impact, the molecular mechanisms underlying ME remain poorly understood. This review synthesizes current evidence on the role of DNA, both nuclear and mitochondrial, in the susceptibility and pathophysiology of ME. We examined genetic predispositions, including familial clustering and candidate gene associations, and highlighted emerging insights from genome-wide and multi-omics studies. Mitochondrial DNA variants and oxidative stress-related damage are discussed in relation to impaired bioenergetics and symptom severity. Epigenetic modifications, particularly DNA methylation dynamics and transposable element activation, are explored as mediators of gene–environment interactions and immune dysregulation. Finally, we explored the translational potential of DNA-based biomarkers and therapeutic targets, emphasizing the need for integrative molecular approaches to advance diagnosis and treatment. Understanding the DNA-associated mechanisms in ME offers a promising path toward precision medicine in post-viral chronic diseases.
Graphical Abstract
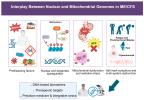
Myalgic Encephalomyelitis (ME), also known as chronic fatigue syndrome (CFS), is a debilitating and heterogeneous disorder marked by persistent fatigue, post-exertional malaise, cognitive impairment, and multisystem dysfunction. Despite its prevalence and impact, the molecular mechanisms underlying ME remain poorly understood. This review synthesizes current evidence on the role of DNA, both nuclear and mitochondrial, in the susceptibility and pathophysiology of ME. We examined genetic predispositions, including familial clustering and candidate gene associations, and highlighted emerging insights from genome-wide and multi-omics studies. Mitochondrial DNA variants and oxidative stress-related damage are discussed in relation to impaired bioenergetics and symptom severity. Epigenetic modifications, particularly DNA methylation dynamics and transposable element activation, are explored as mediators of gene–environment interactions and immune dysregulation. Finally, we explored the translational potential of DNA-based biomarkers and therapeutic targets, emphasizing the need for integrative molecular approaches to advance diagnosis and treatment. Understanding the DNA-associated mechanisms in ME offers a promising path toward precision medicine in post-viral chronic diseases.
Graphical Abstract

