Dolphin
Senior Member (Voting Rights)
CANCER CASE REPORTS - Volume 2; Issue 1, April to June
Pages: 1-4
Date of Publication: 08-Apr-2020
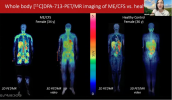
Everything is in The Vagus Nerve: What is The Relationship Between Chronic Fatigue Syndrome (CFS) and Coronavirus?
Author: Pr. Sakhri Selma
Category: Health Care
Abstract:
The vagus nerve, also known as the pneumogastric nerve, is the tenth pair of cranial nerves, involved in many functions of the body.
The sensory vagus nerve contains chemoreceptors sensitive to the presence of pro-inflammatory cytokines.
It innervates the tissues that are often the first points of contact for foreign pathogens, such as the lining of the esophagus, the gastrointestinal lining, the lungs and lymph nodes.
The vagus nerve also innervates most other important organs of the trunk such as the spleen, liver, heart, bladder, and pancreas.
Keywords: Vagus Nerve, Chronic Fatigue Syndrome, Coronavirus
Full Text:
https://www.cancercasereports.com/article_html.php?did=7228&issueno=0