OK, so here's my paper summary previously posted here on the other forum (a couple of tweaks noted):
I think this is the most exciting study since I first came across the hypometabolism (aka dauer)
metabolomic studies and Naviaux's
Oxidative Shielding, CDR (
Cell Danger Response) and
Healing Cycle framework, upon which
this paper builds!
I'm a decades long gradual onset case, with no obvious acute/infectious events and I've not had colds or flu in many years (hmmm ). So viruses have largely been outside of my self-research interest. Hence I've now been reading up on (and around) this HHV-6 topic since Prusty posted the paper. Minus a few days of frustrating energy crashes, of course. I'll try to summarise my understanding (please correct and question), then do my usual of posting a big list of over-excited questions, later on...
To oversimplify (and and probably overstate) the findings:
ME/CFS is caused by *partial* reactivation of human herpes virus 6 DNA in *some* of our cells, which then send a mystery signalling molecule(s) through our blood that shuts down the rest of our cells.
In more detail:
Prusty et al. demonstrated that human cells (U2-OS), cultured in a lab dish and treated to undergo partial HHV-6 reactivation, secreted a substance into the surrounding medium (supernatant) that, when transferred to a second (responder) culture of separate cells (A549), inhibited cellular energy production.
Serum from each of 10 ME/CFS patients produced the exact same effects! Both also gave the second culture of cells an innate immune resistance to infection from influenza-A (H1N1) and HSV-1 viruses.
They say HHV-6, Human Herpes virus, is found in 90-100% of all humans by age 3. So virtually everyone has it and HHV-7 is very similar. Herpes viruses insert their RNA into the nucleus of human cells, becoming a 'latent' infection, without need to retain viral particles anywhere. But HHV-6 is unique in making its code 'chromosomally integrated', directly part of our own DNA strands. In fact, up to 1% of people are born with inherited HHV-6! Having it in every cell.
They used a drug called TSA (trichostatin-A) that's well known to 'reactivate' HHV-6 in cells they had infected previously. They describe the drug as modelling "genetic and environmental stressors". I think this might equate to the initial acute infectious events, etc, that initiate ME/CFS.
This reactivation didn't go as far as making actual functional virus particles, hence they termed it "transactivation". Only non-structural helper proteins are being made inside of the cells, which are causing metabolic changes and mitochondria to splitting into smaller structures (fission). They found that the most indicative of these proteins is U14, which was also detected in the blood of 40% (8 of 20) patient samples.
They still haven't been able to separate the active (fatigue factor) substance(s) from the transferred cell culture liquid (or ME/CFS serum), in order to identify it. But that seems extremely close at hand, now! Prusty claimed in January that they had a candidate and a couple tests in mind already [previous thread].
Some more notes related to all this:
► The laboratory cells used in the experiments are from
cancers. These cell lines are effectively immortal, convenient for standardising lab-work. But they behave a little differently to regular healthy cells, including a strong tenancy towards growth, etc, so exact results aren't proof of behaviour of patient's cell in the body. I.e. ME/CFS
patients are not immune to infections, line Covid-19 [
Twitter]! The cell lines are:
► U2-OS - Human Bone Osteosarcoma Epithelial Cell, 2T line, established 1964 [
microscopyu].
• Their custom U2-OS cells were developed for this 2018 paper [
Nature], with latent (chromosomally integrated) ciHHV-6 in the DNA.
• GFP (green fluorescence protein) was integrated into the mitochondrial DNA (for clear visualisation under microscope) of a second batch of U2-OS cells without ciHHV-6 DNA. These were studied after transfer of supernatant from the ciHHV-6 cells, also.
► A549 - Adenocarcinomic human alveolar basal epithelial cells, established 1972 [
Wikipedia].
• Chosen to be able to support infection by the test viruses.
[
Note: like the majority of human cells, neither cell line can support complete production of HHV-6 virus particles, so are good for studying early stages of reactivation.]
► TSA - histone deacetylase inhibitor trichostatin-A [
Wikipedia] - the reactivation trigger which "models Genetic and environmental stressors".
• An antifungal and antibiotic with possible anti-cancer action (via encouraging apoptosis).
• Inhibits the class I and II mammalian histone deacetylase (HDAC) enzyme family [
Wikipedia]. Interfering with the removal of acetyl groups, altering gene expression.
►
Viral immunity experiment - Comparing effect on (A549) responder cells of being cultured in liquid from (1) trans activated U2-OS cells, (2) ME/CFS patient serum, (3) non-activated U2-OS cells, (4) Control subject serum:
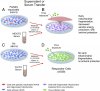
► HHV-6 transactivation's altered enzymes levels in U2-OS cells,
(+) = Increased factors,
(-) = Decreased, from the paper's abstract:
(+)
1-carbon metabolism - AKA methylation cycle.
(+)
dUTPase [
Wikipedia] - produces dUMP (precursor of thymidine), decreases dUTP (avoiding uracil's accidental use in DNA in place of thymine) [
PhosphoSite]. dUTPases (Udeoxyuridine triphosphate nucleotidohydrolases) are key modulators of innate and adaptive immune responses [
NCBI].
(+)
Thymidylate synthase - dUMP to dTMP (thymidine monophosphate - DNA nucleotide) [
Wikipedia]...
(-)
SOD2 (superoxide dismutase 2) - clears reactive oxygen species from mitochonrial energy production. Protects against cell death and inflamatory cytokines.
(-)
PDH (pyruvate dehydrogenase) and other proteins required for mitochondrial oxidation of fatty acid, amino acid, and glucose metabolism.
Expressed levels of many mitochondria associated proteins (enzymes) where changed, as measured by pSILAC experiment (pulsed stable isotope labeling by amino acids in cell culture) some significant ones labelled in [
Fig1 of paper]:
 Note
Note: I think it's only implied that all of these changes in the transactivated cells are transferred via the factor in the cell medium to the second set of cells... Also, I'm not sure how much we should keep in mind that these were cancer cells, with a quite different metabolic resting state that may be affected differently to healthy cells.
► sncRNA-U14 - small noncoding RNA from HHV-6, potent biomarker of recent reactivation, found in 8 of 20 ME/CFS pateint serum (0 of 5 controls). I don't know if it might be present in all ME/CFS cases at below detectable levels [?], but I feel that host cell signals are more likely the main factor (like Naviaux talks about with ATP secretion). List of factors currently being investigated, below...
► Other HHV-6 Viral proteins [
Nature] :
•
U3-U7 - latency associated.
• U14 - transactivation (early stage - which has been found in many other viruses and bacteria).
•
U11 - late stage, needed for making viron (virus particles).
• [Incomplete, could not find a clear list and hard to extract from research papers...]
► Beyond the immediate findings,
the setup used is itself a big deal! Giving an alternative to the nano-needle test for detecting the serum factor. Far less practical in an eventual clinical setting, as is, but allowing reproduction in other experimental laboratories. It's also a model for *
creating* fatigue factor molecules, in a well known cell line that they can pick apart and analyse extremely closely while its doing it:
"
Our mitochondrial reporter-based cell system will provide an opportunity to develop a diagnostic test for ME/CFS as well as provide a platform for further identification of potential factors that define ME/CFS pathophysiology."
►
Herpes viruses (all types) establish lifelong infection via many tricks [
Wikipedia]. E.g.:
• Producing protein mimicking human interleukin 10 (
hIL-10) = cmvIL-10.
Inhibits pro-inflamatory cytokines (IFN-γ, IL-1α, GM-CSF, IL-6 and TNF-α).
• Downregulating
MHC I and II (major histocompatibility complex):
- Prevents them presenting viral antigen proteins from inside to cell surface.
- This stops cytotoxic T lymphocytes identifying the infected cell.
•
HLA-G upregulated to suppress natural killer cells which usually attack cells not presenting MHCs.
►
Roseola [
NHS |
Wikipedia] body rash following fever is how
*initial* HHV-6 (A and B) and HHV-7 infections present in young childhood. Although sometimes asymptomatic, these infections may account for up to ~30% of ER visits for young children with sudden high fever (for up to 5 days).
► Receptor binding for cell entry of virus (and other details), CD = "
Cluster of differentiation":
[
Note: Prusty's said that the receptors are expressed everywhere (to some extent) so the viruses could access all cells.]
•
HHV-6A =>
CD46 [
Wikipedia]
Inhibitory complement receptor (system that enhances antibodies and phagocytic cells).
Also exploited by a strain of measles and group B adenoviruses.
•
HHV-6B =>
CD134 [
PubMed |
Wikipedia]
AKA "OX40". Part of Tumor necrosis factor receptor superfamily, member 4 (TNFRSF4).
Mostly on CD4 T-Cells.
Activation increases cytokine production.
Associated with pathologic cytokine storm in e.g. H5N1 bird flu.
Activation critical for sustained immune response (past first few days).
• HHV-7 =>
CD4 (and some cell surface glyoproteins) [
Wikipedia]
Enters CD4+ T cells (and macrophages, dendritic cells).
Downregulates CD4 a week after infection (so may interfere with HIV, but reactivaties HHV-6).
95% of adults infected by age 2-5 (generally after HHV-6).
20% show virus in blood, 98% in mouth.
►
ME/CFS and HHV-6 on [
HHV-6 foundation]: Diagnosis & antiviral treatment; Chromosomally integrated HHV-6 or
CIHHV-6; Infecting the brain via the Olfactory (nose) Nerve (affecting Limbic system, Hippocampus); reactivated in transplant patients & under stress; resident in sensory ganglia (per VanElzakker's hypothesis), list of key papers (from researchers e.g. Montoya, Learner, Komaroff, etc).
►
Fatigue factor molecule types Prusty has talked about looking for specifically [
YouTube]:
• Mitochondrial metabolites (analysing in conjunction with Naviaux).
• Exosomes containing small non-coding RNAs or proteins. Are these the "
cryptic peptides" [
Twitter]?
• RNA - Cellular or pathogenic (
including U14).
• Antibodies - auto (against self), against viruses.
• Calcium flux - altered inside cells so also outside.
List of questions (for researchers or whomever) to follow...






