Did you see at the end of one graph what is more commonly known as African Sleeping Sickness? I wonder if this is important.With a bit of googling, supplementary materials :
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Identification of actin network proteins, talin-1 and filamin-A, in circulating extracellular vesicles as blood biomarkers...ME/CFS 2019, Eguchi et al
Simon M
Senior Member (Voting Rights)
Comment
Thanks for all the analysis on this thread. Here are some additional points from me.
I guess the main point is that the full text doesn't really live up to the big claims in the abstract. But some of the findings are interesting.
The conclusion is more accurate, so I'll start with that, then track back to the claims made earlier.
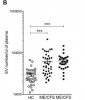
That holds vs healthy controls, see fig 1B for the two ME/CFS samples (ME/CFS 1=39, ME/CFS 2=30, healthy controls HC=36), p<0.001 for both. Third ME/CFS sample of 30 vs 20 HC also p<0.001 in supplementary material, which I can't find.[We] revealed that circulating EV levels are significantly increased in ME/CFS Patients.
Note that these findings are consistent with the Spanish pilot study (10 ME/CFS vs 5 HC): "We found that the amount of EV-enriched fraction was significantly higher in CFS/ME subjects than in HCs (p = 0.007)".
Somewhat overstating things. Elsewhere they talk about how they did 2 independent analyses, but since one is 3 ME/CFS vs 3 HC and the other 4 each of ME/CFS, depression and idiopathic chronic fatigue this is far from convincing. Even so, it helps that the top proteins that differed in amount for ME/CFS vs others were pretty similar in both analyses. It’ll be very interesting to see if this replicates.These EVs contain a specific protein cargo, particularly actin network proteins and 14-3-3 family proteins, which represent novel-specific ME/CFS biomarkers and can distinguish this condition from ICF and clinical depression, which are two highly challenging differential diagnoses in the clinical arena.
That is suitably reasonable.Future studies including larger cohorts that would allow for matching the various conditions by key variables such as age and gender as well as external validation studies are warranted.
The novel findings of this study may open new windows to reveal ME/CFS pathogenic mechanisms and may aid in the development of better ME/CFS biomarkers and effective therapies.
Now the stuff that I feel isn't supported.
From the highlights section:
Factually correct, but more useful is to look at the data in Fig 1B shown above - there is a lot of overlap between patients and controls (though the average difference between the 2 groups is unusually large).[Area under the curve, AUC] for circulating EVs was 0.802 allowing correct diagnosis in 90-94% of ME/CFS.
An AUC of 0.8 isn't that great given that a coin toss achieves an AUC of 0.5. Presumably, the 90-94% correct diagnosis rate is based on choosing a good EV threshold for this study's data, which might not work out so well in an independent cohort. And, if I've understood this right, an EV threshold identifying 90-94% of patients (high sensitivity) will then wrongly identify quite a high proportion of of healthy controls as having ME/CFS (low specificity). There is always a trade-off between sensitivity and specificity.
Also, a biomarker is only really useful for diagnosis if it distinguishes from similar-looking diseases, and there was no significant difference vs depression or chronic fatigue.
The skeletal claim is a bit of a push: the analysis of the proteomic results talked about proteins involved in regulation of the actin cytoskeleton, and the basis for skeletal regulation is more tenuous.Proteins in actin skeletal regulation and EB virus infection were identified in ME/CFS patients.
Actin network proteins also play important roles in the skeletal muscle as follows: 1) talin 1 regulates
the stability of myotendinous junctions [muscle-tendon junctions, not part of the key actin-myosin interaction that leads to muscle contraction] through the vinculin-talin-integrin system in skeletal
muscle (Conti et al., 2008); and 2) human serum gelsolin [note, not EV gelsolin, which is measured here] is mainly derived from skeletal muscle (Kwiatkowski et al., 1988).
Note that myosin-9 was one of the top proteins found to differ between mecfs and other groups, but this is a cytoskeleton protein, different from the myosin protein that makes up a major part of skeletal muscle.
That said, their finding of a link to the cytoskeleton is intriguing, I just wish they hadn’t overegged things.
Going to leave it there for tonight
Attachments
Last edited:
In other words, low specificity.Also, a biomarker is only really useful for diagnosis if it distinguishes from similar-looking diseases, and there was no significant difference vs depression or chronic fatigue.
mariovitali
Senior Member (Voting Rights)
Did you see at the end of one graph what is more commonly known as African Sleeping Sickness? I wonder if this is important.
Yes and perhaps there are more. Please see the following image from the paper :

If you open the image into a new tab you will get a larger version of it (works like this on my browser, Chrome)
-THBS1 aka TSP1 cc @ScottTriGuy (24th from the bottom. Figure B) which was mentioned by Alain Moreau and was also identified along with other genes through Machine Learning, related to Phagocytosis / apoptotic cell clearance (hypothesis)
-ALB Gene , for Albumin. Albumin is also being identified by Machine Learning , unfortunately i have no idea why this is so. Nacul et. al found a statistically significant difference on albumin between severely ill vs non-severely ill ME patients :
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6627354/
Actin filaments and actin reorganization is not a new topic in ME/CFS and there are many posts on PR for this topic. Phagocytosis : of course i do not know if this is applicable to ME/CFS but nevertheless i have shown a slide at my EUROMENE presentation that shows TSP1 (THBS1) and also mentions actin. Again, i do not know whether these are applicable to the findings of this paper :

I am sure there will be fascinating things here once we start probing. The reason I am interested in African Sleeping Sickness is the finding that Ron Davis and team made that our micro RNA is a match for it, and the symptoms are often ME symptoms ... they sleep, but mostly and badly during the day, and even worse and even less at night. This more or less matches the circadian reversal we see in many ME patients. Yet we do not have a known trypanosome infection.Yes and perhaps there are more.
An AUC of 0.8 isn't that great given that a coin toss achieves an AUC of 0.5. Presumably, the 90-94% correct diagnosis is based on choosing a good EV threshold for this study's data, which might not work out so well in an independent cohort. And, if I've understood this right, an EV threshold identifying 90-94% of patients will then wrongly identify quite a high proportion of of healthy controls as having ME/CFS.
I think medical people should be encouraged to quote accuracy Precision and Recall scores (or F1) which give a better picture of what is happening in terms of false positives and true positives.
https://towardsdatascience.com/accuracy-precision-recall-or-f1-331fb37c5cb9
There are also questions around how the threshold is chosen and whether the data used to choose a threshold is included in any metrics given.
That said I tend to think that the obsession with a biomarker is perhaps wrong anyway. At this stage interesting differences (which can overlap as distributions such as in the plots you include) seem interesting.
One thing I would like to see is multiple samples from the same patient taken over time with measures of severity and exertion. Does a change in the measures represent something in terms of how people feel and can that be measured.
Jonathan Edwards
Senior Member (Voting Rights)
That said I tend to think that the obsession with a biomarker is perhaps wrong anyway. At this stage interesting differences (which can overlap as distributions such as in the plots you include) seem interesting.
I absolutely agree. To think that the reason for doing studies like this is to find an instant biomarker is naive and blinkered. Rheumatoid factor was never a very useful biomarker for RA but it led us to understand how to treat the disease.
Most people, regardless of cohort, had a CRP value of less that 0.05 mg/dL (0.5 mg/L), with just a small number of outliers (the outliers being both healthy controls and PwME).
The correlation in that graph does look like it may be highly influenced by outliers.
Although I was wondering given the number outside of the main cluster with low CRP which included both heathy and PwME could this be due to some other factor?
Lots of causes of inflammation e.g. coronary heart disease could account for CRP outliers with and without ME/CFS.
This study found a lot of variability in CRP in people both with and without coronary artery disease. Here's the graph showing CRP results taken multiple times for 25 individual people without coronary artery disease.

Check out the scale of that graph - CRP is reported in mg/L. Then check out the graph from this study again (below), bearing in mind that the 3 mg/L on the graph above equals the 0.3 mg/dL in the graph below. The variability found in this study does not appear to be in any way abnormal. If anything, I would have expected more variation.

This study found a lot of variability in CRP in people both with and without coronary artery disease. Here's the graph showing CRP results taken multiple times for 25 individual people without coronary artery disease.

Check out the scale of that graph - CRP is reported in mg/L. Then check out the graph from this study again (below), bearing in mind that the 3 mg/L on the graph above equals the 0.3 mg/dL in the graph below. The variability found in this study does not appear to be in any way abnormal. If anything, I would have expected more variation.

Last edited:
This paper specifies skeletal muscle. Maybe the Australians were on to something with the calcium channelopathy theory. But the tie to EV escapes me. Are EVs remnants or blebs from skeletal muscle cells?
I think the calcium channelopathy theory was badly dented by Wenzhong Xiao at the OMF Stanford Conference a few years ago - there was no supporting evidence from the genetic work. I.e. people with ME were no more likely to have genetic problems, linked to calcium channelopathy, than healthy controls.
I think I've read that pretty much all cells produce exosomes (EDIT RED BLOOD CELLS DO PRODUCE EXOSOMES - https://www.hindawi.com/journals/bmri/2019/2045915/ ETC.). The role of exosomes in disease(s) is really only starting to be researched; Hanson is looking at micro-RNAs. Interesting that these researchers are finding proteins with specific functions - are these proteins the scaffolding of cells?
Last edited:
Hoopoe
Senior Member (Voting Rights)
these proteins the scaffolding of cells?
I understood that some of these proteins are involved in creating the inner structure of cells as well as the cell membrane. Altered cell deformability could have something to do with these proteins. There were other functions too that I forgot and didn't understand.
At first I couldn't understand what these proteins could possibly have to do with ME/CFS. If the cell deformability findings are correct then they might fit quite well.
The Akt/mTOR related stuff has come up before in a variety of other papers but that could mean less than one would initially think. It could be because it's such a central pathway that it's hard to not find it somehow altered.
Kitty
Senior Member (Voting Rights)
When I read research like this it always strikes me that the 'something in the blood' mystery is going to prove hard to unpick until we find ways to study the process of PEM.
If the fabled Something is found in everybody's blood because it's perfectly normal, and the real problem is that it's triggering an abnormal reaction, then we could search for it till Kingdom Come and still get nowhere.
(Might possibly be evident that I'm feeling a bit grumpy today. )
)
If the fabled Something is found in everybody's blood because it's perfectly normal, and the real problem is that it's triggering an abnormal reaction, then we could search for it till Kingdom Come and still get nowhere.
(Might possibly be evident that I'm feeling a bit grumpy today.
When I read research like this it always strikes me that the 'something in the blood' mystery is going to prove hard to unpick until we find ways to study the process of PEM.
If the fabled Something is found in everybody's blood because it's perfectly normal, and the real problem is that it's triggering an abnormal reaction, then we could search for it till Kingdom Come and still get nowhere.
(Might possibly be evident that I'm feeling a bit grumpy today.)
I absolutely agree. To think that the reason for doing studies like this is to find an instant biomarker is naive and blinkered. Rheumatoid factor was never a very useful biomarker for RA but it led us to understand how to treat the disease.
I think the approach suggested by @Jonathan Edwards and @Adrian is interesting. I.e. if you examine the contents of the exosomes then this may provide insight into the disease mechanism. E.g. there's some information here which indicates that the exosomes may contain actin - possible insight into disease mechanism?
If you consider what the effect of something in the blood is, then this might help identify what it is - e.g. it appears to cause mitochondrial fragmentation (Bhupesh Prusty), what causes mitochondrial fragmentation?
Plus if this link to red blood cell deformability is correct, then there may be more incentive to identify the something in the blood.
Reading the paper as a whole I am not very impressed. The introduction, discussion and data presentation do not have the ring of people with a deep understanding of the problem. There is no mention of blinding of samples and the way repeated cohorts have been used does not look to have been done in a way to ensure bias is avoided.
My thought is that extracellular vesicles of this sort are basically cell debris - bits of those cells that have fallen apart. The most likely cells to fall apart and give rise to vesicles in venous blood are neutrophils. I think there may be a sample handling problem that might produce systematic bias. It is also quite possible that the different patterns of activity that occur in PWME have an effect on the number of senescent neutrophils in plasma.
The number of vesicles certainly does not give a diagnostic test since there is major overlap. Most ME patients fell within a normal range constructed from controls. The data on specific proteins looks limited and I suspect was not repeated on a second test cohort.
Very interesting; exosomes are my latest big hope!
So the pattern of activity in people with ME is different (activity levels lower) so some changes can be due to that!
I think Maureen Hanson presented data (OMF - September this year?) showing the number of the smallest EVs (exosomes) was significantly increased in ME. However, I can't remember much about the differences in contents - Hanson's group tested for microRNAs; the levels of some of these were altered. E.g. in healthy controls the levels of some micrRNAs were related; if one was high then another was high etc. ---. However, in people with ME the relationship between the levels of these microRNAs was reversed - if one was high then another was low---
Difficult to see where to go, research wise, in this disease.
Hopefully we can find something that gives insight into the disease mechanism (biomarker) regardless of whether it is diagnostic.
Some things have thresholds, below a certain amount is one response, sometimes a non-response, over that is another due to changes in molecular switches. This might have that kind of effect. However in the case of some chemical sensitivities, it looks like under a certain amount has no abnormal response, but over that has an increasing response according to dosage. This happens because compensating mechanisms, such as glutathione supply (and I am not just talking about the liver) are overwhelmed with respect to substances like salicylates.Isn't it possible that it's the way our cells react to " something in the blood" that matters, not the absolute level of the substance/ substances?
To my understanding signals often cross some kind of threshold to be important. A low amount of something in the blood might not trigger problems, but above an as yet unidentified threshold it could be disastrous. The question in my thinking on this is does it have a worsening effect over that threshold? I would say almost certainly yes, but that is an inference.
Finding a link between mitochondrial function and some of these molecules would seem to be very important. Which of them could impede mitochondrial function? We might not even have discovered the mechanism yet, its not like we know all the biochemistry in human cells, its a work in progress.
Last edited:
rvallee
Senior Member (Voting Rights)
Pretty reasonable. The problem here is likely to be non-linear. There is a reaction to something that is out of proportion to initial conditions and if it happens entirely within cells we just don't have the tools to make it out yet.Isn't it possible that it's the way our cells react to " something in the blood" that matters, not the absolute level of the substance/ substances?
Non-linear problems are super fun in physics because of an abundance of mathematical tools, but in medicine they basically force a dead-end unless there is widespread acceptance that it's worth pursuing until it's solved, with funding to match. Those problems are incredibly hard, the very stuff of what science is all about, but it needs technology to catch up and give us clarity. Which is what happened with the human genome project, technology was built. Early on the problem seemed impossible and with early technology would have taken centuries. Over half of it was done in the last year or so.
Technological progress seems to be about 90% of what makes medicine progress at all, if not more. Without constant technological progress medicine would have almost completely stalled decades ago. We're basically like the field of astronomy waiting for the invention of the telescope. Though more like trying to get the telescope built but being refused institutional support because "there is nothing to see beyond the celestial spheres", or something like that.
Kitty
Senior Member (Voting Rights)
Technological progress seems to be about 90% of what makes medicine progress at all, if not more.
Presumably, there's also a considerable lag in learning how to use it to best advantage too? Not to speak of restricted access, exorbitant costs, etc. That's what cheers me about Dr Davis's approach at Stanford: they look for cheap, accessible options rather than trying to design a new multi-million dollar machine.
I think the calcium channelopathy theory was badly dented by Wenzhong Xiao at the OMF Stanford Conference a few years ago - there was no supporting evidence from the genetic work. I.e. people with ME were no more likely to have genetic problems, linked to calcium channelopathy, than healthy controls.
I think I've read that pretty much all cells produce exosomes (EDIT RED BLOOD CELLS DO PRODUCE EXOSOMES - https://www.hindawi.com/journals/bmri/2019/2045915/ ETC.). The role of exosomes in disease(s) is really only starting to be researched; Hanson is looking at micro-RNAs. Interesting that these researchers are finding proteins with specific functions - are these proteins the scaffolding of cells?
@duncan I noticed something online re intracellular calcium regulation and actin - around when I posted my previous comment. So I Googled for a few seconds just now and found this "A Tripartite Interaction Among the Calcium Channel α1- and β-Subunits and F-Actin Increases the Readily Releasable Pool of Vesicles and Its Recovery After Depletion" [https://www.frontiersin.org/articles/10.3389/fncel.2019.00125/full].
So maybe that was your point in your previous post - i.e. "Griffith's group are claiming intracellular calcium regulation is impaired in ME" is this linked to actin --- maybe.
Don't think I'm even going to try to understand this just now!
Mithriel
Senior Member (Voting Rights)
I think the calcium channelopathy theory was badly dented by Wenzhong Xiao at the OMF Stanford Conference a few years ago - there was no supporting evidence from the genetic work. I.e. people with ME were no more likely to have genetic problems, linked to calcium channelopathy, than healthy controls.
If ME is a channelopathy, it is unlikely to be genetic in orgin. There seems no reason to suppose you can't acquire a channelopathy from autoimmunity, environmental factors or infection damage.
Some cancers cause an autoimmunity which mimics genetic movement disorders.