Long COVID is associated with extensive in-vivo neuroinflammation on [18F]DPA-714 PET (Preprint)
Denise Visser, Sandeep S.V. Golla, Sander C.J. Verfaillie, Emma M. Coomans, Roos M. Rikken, Elsmarieke M. van de Giessen, Marijke E. den Hollander, Anouk Verveen, Maqsood Yaqub, Frederik Barkhof, Janneke Horn, Bart Koopman, Patrick Schober, Dook W. Koch, Robert C. Schuit, Albert D. Windhorst, Michael Kassiou, Ronald Boellaard, Michele van Vugt, Hans Knoop, Nelleke Tolboom, Bart N.M. van Berckel
A significant number of COVID-19 patients develop 'long COVID', a condition defined by long-lasting debilitating, often neurological, symptoms. The pathophysiology of long COVID is unknown.
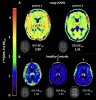
Here we present in-vivo evidence of widespread neuroinflammation in long COVID, using a quantitative assessment, [18F]DPA-714 PET, in two long COVID patients. We reanalyzed historical data from three matched healthy control subjects, for comparison purposes. Both patients with long COVID had widespread increases in [18F]DPA-714 binding throughout the brain. Quantitative measures of binding (BPND values) were increased on average by 121% and 76%, respectively.
This implicates profound neuroinflammation in the pathophysiology of long COVID.
Link | PDF
Denise Visser, Sandeep S.V. Golla, Sander C.J. Verfaillie, Emma M. Coomans, Roos M. Rikken, Elsmarieke M. van de Giessen, Marijke E. den Hollander, Anouk Verveen, Maqsood Yaqub, Frederik Barkhof, Janneke Horn, Bart Koopman, Patrick Schober, Dook W. Koch, Robert C. Schuit, Albert D. Windhorst, Michael Kassiou, Ronald Boellaard, Michele van Vugt, Hans Knoop, Nelleke Tolboom, Bart N.M. van Berckel
A significant number of COVID-19 patients develop 'long COVID', a condition defined by long-lasting debilitating, often neurological, symptoms. The pathophysiology of long COVID is unknown.
Here we present in-vivo evidence of widespread neuroinflammation in long COVID, using a quantitative assessment, [18F]DPA-714 PET, in two long COVID patients. We reanalyzed historical data from three matched healthy control subjects, for comparison purposes. Both patients with long COVID had widespread increases in [18F]DPA-714 binding throughout the brain. Quantitative measures of binding (BPND values) were increased on average by 121% and 76%, respectively.
This implicates profound neuroinflammation in the pathophysiology of long COVID.
Link | PDF