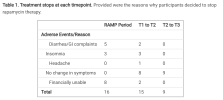
Yeah, that's pretty clear, thanks @ME/CFS Skeptic. There's really nothing there beyond what you'd expect from a placebo, natural fluctuations, and more than half of your cohort (probably the cohort with the least improvement) dropping out.but it does give a ceiling to how large the effect might be.
I did like the ATG13 work. I haven't read this study and I'm hazy on the previous work now, but do people think there is a way that finding might still be worth investigating?