You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
mtDNA deletion [...] with < 10 % heteroplasmy in muscle and isolated complex-V dysfunction misinterpreted as [CFS] over 21-years, 2025, Finsterer+
- Thread starter forestglip
- Start date
The patient is a 52-year-old woman who was diagnosed with myalgic ME/CFS at the age of 25 after an infection with parvovirus B19 and has not been the same since. For the past twenty-one years, she has suffered from chronic fatigue, exercise intolerance, and post-exertional malaise and has been mostly housebound and often bedbound (up to 22 h per day).
The patient presented is interesting in several respects. Firstly, the m.8753_16566del variant has not previously been described as a cause of MID [mitochondrial disorder]. Second, the mtDNA deletion caused isolated complex-V dysfunction. Third, the mtDNA deletion manifested phenotypically despite a heteroplasmy rate of <10 % in muscle. Fourth, MID remained undetected for 21-years. Fifth, the mtDNA deletion manifested only in skeletal muscle. Sixth, the mtDNA variant first became symptomatic after a parvovirus infection. An isolated complex-V insufficiency was previously described at least in lymphocytes of ME/CFS patients. [5]
[5] is: An Isolated Complex V Inefficiency and Dysregulated Mitochondrial Function in Immortalized Lymphocytes from ME/CFS Patients (Missailidis et al, 2020, Int. J. Mol. Sci.) (S4ME link)
Interesting to see how this is framed as a "misinterpretation" or "mimicking" of ME/CFS, when very clearly the patient has ME/CFS (qualifying under the usual syndromic definitions, as quoted by Forestglip above). Why not simply speculate that this could be a potential cause for ME/CFS?
Also fascinating that she would have had muscle biopsies, endocardial biopsy, NK function tests and CPET, yet has not had a simple brain MRI despite "Since the age of 34, she had recurrent strokelike symptoms".
Key points —
KSS is Kearns-Sayre Syndrome.
Also fascinating that she would have had muscle biopsies, endocardial biopsy, NK function tests and CPET, yet has not had a simple brain MRI despite "Since the age of 34, she had recurrent strokelike symptoms".
Key points —
A muscle biopsy at the age of 39 years revealed an increased lipid content and a predominance of type II muscle fibers, which was considered a non-specific finding.
VO2 max tests showed a drastic reduction in aerobic capacity.
KSS is Kearns-Sayre Syndrome.
mtDNA analysis from muscle at 46 years of age revealed the m.8753_16,566 deletion with <10 % heteroplasmy. The deletion was larger than in patients with KSS but encompassed the entire ATP6 gene associated with complex-V of the respiratory chain. An abnormal complex-V band was detected in the MitoFIND assay, indicating that the mutation was a germline mtDNA deletion
intravenous immunoglobulins had a transient positive effect.
the mtDNA deletion caused isolated complex-V dysfunction […] manifested phenotypically despite a heteroplasmy rate of <10 % in muscle […] manifested only in skeletal muscle […] first became symptomatic after a parvovirus infection.
Utsikt
Senior Member (Voting Rights)
I hope we don’t get to a point where anyone with ‘biological’ changes don’t have ME/CFS.Interesting to see how this is framed as a "misinterpretation" or "mimicking" of ME/CFS, when very clearly the patient has ME/CFS (qualifying under the usual syndromic definitions, as quoted by Forestglip above). Why not simply speculate that this could be a potential cause for ME/CFS?
Murph
Senior Member (Voting Rights)
Whenever I read about people having problems with the various complexes in the mitochondrial chain I think about Hwang and WASF3. He found in his patient that WASF3 inhibited two complexes joining together to make a supercomplex, which would run more efficiently (and then detected high wasf3 in a wider group). Maybe the variation in mecfs is about different ways the chain is broken?
The only problem I have with a mitochondrial centric view of me/cfs is that I'd have guessed we would have found the problem by now if it was indeed usually in the mitochondria.
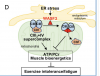
The only problem I have with a mitochondrial centric view of me/cfs is that I'd have guessed we would have found the problem by now if it was indeed usually in the mitochondria.

Utsikt
Senior Member (Voting Rights)
How easy is it to study mitochondria, and how much do we know about them? I’m assuming their functionality is highly dependent on the environment they are in - so isolating them might produce different behaviours?The only problem I have with a mitochondrial centric view of me/cfs is that I'd have guessed we would have found the problem by now if it was indeed usually in the mitochondria.
True, ME could involve a mitochondrial problem in only a few specialized brain cells. Very hard to find that unless you know what to look for (and where). Moreover, it might not show up in autopsy samples, or even living cells without the environmental factors that lead to ME.I’m assuming their functionality is highly dependent on the environment they are in - so isolating them might produce different behaviours?
DMissa
Senior Member (Voting Rights)
"mtDNA analysis from muscle at 46 years of age revealed the m.8753_16,566 deletion with <10 % heteroplasmy. The deletion was larger than in patients with KSS but encompassed the entire ATP6 gene associated with complex-V of the respiratory chain. An abnormal complex-V band was detected in the MitoFIND assay, indicating that the mutation was a germline mtDNA deletion"
One of our ME PhD students is looking at this gene (amongst other things)!
One of our ME PhD students is looking at this gene (amongst other things)!
DMissa
Senior Member (Voting Rights)
How easy is it to study mitochondria, and how much do we know about them? I’m assuming their functionality is highly dependent on the environment they are in - so isolating them might produce different behaviours?
Can study them inside live samples from patients - easier or harder depending on the tissue of interest. We have done it in circulating immune cells, primary fibroblast lines and immortalised cell lines. Others have done it on immune cell subsets and muscle biopsies
poetinsf
Senior Member (Voting Rights)
This is an important point that needs to be debated. I've heard that conditions like IH, CCI or even unidentified bone-infection can mimic ME/CFS. The problem is that those are treatable conditions. I've even met someone who suffered from hemochromatosis (?) or something and cried river saying that his life was now over because he had ME/CFS, and then got cured 3 years later after getting treated properly. I don't know the mitochondrial condition in this paper is treatable or not, but it apparently explains the symptoms. And the syndrome definition requires that the symptoms are not explained by other conditions.Interesting to see how this is framed as a "misinterpretation" or "mimicking" of ME/CFS, when very clearly the patient has ME/CFS (qualifying under the usual syndromic definitions, as quoted by Forestglip above). Why not simply speculate that this could be a potential cause for ME/CFS?
I think it just goes to show the importance of getting diagnosed properly. There is no reason to suffer needlessly if it is treatable. That's not same thing as saying that it is not ME/CFS if it has a biological cause. ME/CFS will eventually prove to have a biological cause, I'm sure. It's just that it is not known at the moment.
Kitty
Senior Member (Voting Rights)
Posted on behalf of @MSEsperanza:
I'm afraid that omission aligns well with at least one of the authors having been associated with using paper mills for publishing.
Thanks to Nick Brown https://bsky.app/profile/steamtraen.hopto.org for digging out the pubpeer comment on an unrelated paper by Finsterer:
https://pubpeer.com/publications/0122380B6FAE8AFE8625052182E72D
I thought just Finsterer's rate and variety of publications was strange, also those in the more unsuspicious journals mostly tend to be letters:
https://orcid.org/0000-0003-2839-7305
It's still possible that there is some sensible stuff in the paper, just maybe better to remain extra-skeptical in this case.
I'm afraid that omission aligns well with at least one of the authors having been associated with using paper mills for publishing.
Thanks to Nick Brown https://bsky.app/profile/steamtraen.hopto.org for digging out the pubpeer comment on an unrelated paper by Finsterer:
https://pubpeer.com/publications/0122380B6FAE8AFE8625052182E72D
I thought just Finsterer's rate and variety of publications was strange, also those in the more unsuspicious journals mostly tend to be letters:
https://orcid.org/0000-0003-2839-7305
It's still possible that there is some sensible stuff in the paper, just maybe better to remain extra-skeptical in this case.
