Sly Saint
Senior Member (Voting Rights)
Orthostatic Intolerance after COVID-19 Infection: Is Disturbed Microcirculation of the Vasa Vasorum of Capacitance Vessels the Primary Defect?
Wirth and Lohn
Abstract
:
Following COVID-19 infection, a substantial proportion of patients suffer from persistent symptoms known as Long COVID. Among the main symptoms are fatigue, cognitive dysfunction, muscle weakness and orthostatic intolerance (OI).
These symptoms also occur in myalgic encephalomyelitis/chronic fatigue (ME/CFS). OI is highly prevalent in ME/CFS and develops early during or after acute COVID-19 infection.
The causes for OI are unknown and autonomic dysfunction is hypothetically assumed to be the primary cause, presumably as a consequence of neuroinflammation. Here, we propose an alternative, primary vascular mechanism as the underlying cause of OI in Long COVID.
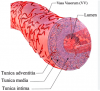
We assume that the capacitance vessel system, which plays a key role in physiologic orthostatic regulation, becomes dysfunctional due to a disturbance of the microvessels and the vasa vasorum, which supply large parts of the wall of those large vessels.
We assume that the known microcirculatory disturbance found after COVID-19 infection, resulting from endothelial dysfunction, microthrombus formation and rheological disturbances of blood cells (altered deformability), also affects the vasa vasorum to impair the function of the capacitance vessels. In an attempt to compensate for the vascular deficit, sympathetic activity overshoots to further worsen OI, resulting in a vicious circle that maintains OI. The resulting orthostatic stress, in turn, plays a key role in autonomic dysfunction and the pathophysiology of ME/CFS.
https://www.mdpi.com/1648-9144/58/12/1807
Wirth and Lohn
Abstract
:
Following COVID-19 infection, a substantial proportion of patients suffer from persistent symptoms known as Long COVID. Among the main symptoms are fatigue, cognitive dysfunction, muscle weakness and orthostatic intolerance (OI).
These symptoms also occur in myalgic encephalomyelitis/chronic fatigue (ME/CFS). OI is highly prevalent in ME/CFS and develops early during or after acute COVID-19 infection.
The causes for OI are unknown and autonomic dysfunction is hypothetically assumed to be the primary cause, presumably as a consequence of neuroinflammation. Here, we propose an alternative, primary vascular mechanism as the underlying cause of OI in Long COVID.
We assume that the capacitance vessel system, which plays a key role in physiologic orthostatic regulation, becomes dysfunctional due to a disturbance of the microvessels and the vasa vasorum, which supply large parts of the wall of those large vessels.
We assume that the known microcirculatory disturbance found after COVID-19 infection, resulting from endothelial dysfunction, microthrombus formation and rheological disturbances of blood cells (altered deformability), also affects the vasa vasorum to impair the function of the capacitance vessels. In an attempt to compensate for the vascular deficit, sympathetic activity overshoots to further worsen OI, resulting in a vicious circle that maintains OI. The resulting orthostatic stress, in turn, plays a key role in autonomic dysfunction and the pathophysiology of ME/CFS.
https://www.mdpi.com/1648-9144/58/12/1807
Last edited by a moderator: