SARS-CoV-2 spike protein induces endothelial inflammation via ACE2 independently of viral replication
Montezano, Augusto C.; Camargo, Livia L.; Mary, Sheon; Neves, Karla B; Rios, Francisco J; Stein, Ross; Lopes, Rheure A.; Beattie, Wendy; Thomson, Jacqueline; Herder, Vanessa; Szemiel, Agnieszka M.; McFarlane, Steven; Palmarini, Massimo; Touyz, Rhian M.
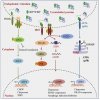
COVID‑19, caused by SARS‑CoV‑2, is a respiratory disease associated with inflammation and endotheliitis. Mechanisms underling inflammatory processes are unclear, but angiotensin converting enzyme 2 (ACE2), the receptor which binds the spike protein of SARS‑CoV‑2 may be important.
Here we investigated whether spike protein binding to ACE2 induces inflammation in endothelial cells and determined the role of ACE2 in this process. Human endothelial cells were exposed to SARS‑CoV‑2 spike protein, S1 subunit (rS1p) and pro‑inflammatory signaling and inflammatory mediators assessed. ACE2 was modulated pharmacologically and by siRNA. Endothelial cells were also exposed to SARS‑CoV‑2. rSP1 increased production of IL‑6, MCP‑1, ICAM‑1 and PAI‑1, and induced NFkB activation via ACE2 in endothelial cells. rS1p increased microparticle formation, a functional marker of endothelial injury. ACE2 interacting proteins involved in inflammation and RNA biology were identified in rS1p‑treated cells. Neither ACE2 expression nor ACE2 enzymatic function were affected by rSP1. Endothelial cells exposed to SARS‑CoV‑2 virus did not exhibit viral replication.
We demonstrate that rSP1 induces endothelial inflammation via ACE2 through processes that are independent of ACE2 enzymatic activity and viral replication. We define a novel role for ACE2 in COVID‑19‑ associated endotheliitis.
Link | PDF (Nature Scientific Reports)
Montezano, Augusto C.; Camargo, Livia L.; Mary, Sheon; Neves, Karla B; Rios, Francisco J; Stein, Ross; Lopes, Rheure A.; Beattie, Wendy; Thomson, Jacqueline; Herder, Vanessa; Szemiel, Agnieszka M.; McFarlane, Steven; Palmarini, Massimo; Touyz, Rhian M.
COVID‑19, caused by SARS‑CoV‑2, is a respiratory disease associated with inflammation and endotheliitis. Mechanisms underling inflammatory processes are unclear, but angiotensin converting enzyme 2 (ACE2), the receptor which binds the spike protein of SARS‑CoV‑2 may be important.
Here we investigated whether spike protein binding to ACE2 induces inflammation in endothelial cells and determined the role of ACE2 in this process. Human endothelial cells were exposed to SARS‑CoV‑2 spike protein, S1 subunit (rS1p) and pro‑inflammatory signaling and inflammatory mediators assessed. ACE2 was modulated pharmacologically and by siRNA. Endothelial cells were also exposed to SARS‑CoV‑2. rSP1 increased production of IL‑6, MCP‑1, ICAM‑1 and PAI‑1, and induced NFkB activation via ACE2 in endothelial cells. rS1p increased microparticle formation, a functional marker of endothelial injury. ACE2 interacting proteins involved in inflammation and RNA biology were identified in rS1p‑treated cells. Neither ACE2 expression nor ACE2 enzymatic function were affected by rSP1. Endothelial cells exposed to SARS‑CoV‑2 virus did not exhibit viral replication.
We demonstrate that rSP1 induces endothelial inflammation via ACE2 through processes that are independent of ACE2 enzymatic activity and viral replication. We define a novel role for ACE2 in COVID‑19‑ associated endotheliitis.
Link | PDF (Nature Scientific Reports)