ThisI have suspected for maybe two decades and a half that our disturbed sleep might be associated with altered circadian patterns of many metabolites. This was my concern in the late 90s when early morning urinary metabolites were first being looked at. Change the time, or have patients with altered circadian patterns, and you might get different results. We cannot yet be sure that our results will be stable over the course of a sleep-wake cycle. This needs to be established, or an optimal time of day or sleep-wake cycle needs to be determined.
[Edited to correct how long its been.]
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Urine Metabolomics Exposes Anomalous Recovery after Maximal Exertion in Female ME/CFS Patients 2023, Glass, Hanson et al
- Thread starter Sly Saint
- Start date
And/or some problem with clearing them, particularly overnight during sleep.I have suspected for maybe two decades and a half that our disturbed sleep might be associated with altered circadian patterns of many metabolites.
jnmaciuch
Senior Member (Voting Rights)
A screen like this will probably not ever get sufficient funding for a replication attempt. It’s just seen as a hypothesis generating exercise, so if already done once no funding body sees the point in doing it again (even the very rare private funds specifically for replication)This got a lot of attention 2-3 years ago when it came out.
Do we have any much news on a replication attempt or similar?
V.R.T.
Senior Member (Voting Rights)
That's crazy! Surely the lack of expected change seen here could have diagnostic purposes? And theorising around it would be much easier if people could be confident in it being replicable.A screen like this will probably not ever get sufficient funding for a replication attempt. It’s just seen as a hypothesis generating exercise, so if already done once no funding body sees the point in doing it again (even the very rare private funds specifically for replication)
The way people talk about capital s Science compared to the reality of how scientific research works in the real world is so disappointing.
Last edited:
jnmaciuch
Senior Member (Voting Rights)
I'll be honest, the chances of this figure reflecting a real biological phenomenon of no change after exercise is so small I would dismiss it outright.Surely the lack of expected change seen here could have diagnostic purposes? And theorising around it would be much easier if people could be confident in it being replicable.
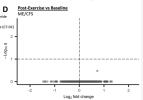
What this graph means is that either the intragroup variability for every single metabolite was so wild that there was no way for any data point to reach significance, or there was a problem in the statistical analysis (either human error while running the code, an inappropriate model for the data, [edit: or incorrectly calculating the q value]). Even running the same sample in two different batches won't give you a graph like this.
The Hanson lab didn't run these samples, they were sent off to a company, so there might have been no way to ask for the samples to be rerun to confirm whether there was a technical issue in the sample processing that made things go funny.
So to your point, it would be ideal to get funding for another study to redo this and see what the actual changes were, but given that there actually have been other urinalysis studies done in ME/CFS and no smoking gun, I think a funding body wouldn't see the point.
Last edited:
jnmaciuch
Senior Member (Voting Rights)
Actually, I'm positive it was an error in the code because definitionally for a q-value by BH you should have a number of false positives determined by your false discovery rate above the dotted line. All the plots in that figure seem to have the same issue.
I don't think this is correct. For example, if all the findings are actually null, which would result in a uniform distribution of p-values between 0 and 1, there's a good chance nothing will pass the FDR threshold.definitionally for a q-value by BH you should have a number of false positives determined by your false discovery rate above the dotted line.
I verified with some quick code which output a minimum q-value of 0.31 if the p-values aren't skewed at all to low values:
Python:
In [1]: import numpy as np
...: from scipy.stats import false_discovery_control
...:
...: ps = np.random.uniform(0, 1, 10000)
...:
...: fdr_ps = false_discovery_control(ps, method='bh')
...:
...: np.min(fdr_ps)
Out[1]: 0.31231890557870123There still might be an issue somewhere, but it's theoretically possible with regard to q-value.
jnmaciuch
Senior Member (Voting Rights)
Yes, sorry for not clarifying, this was with the assumption that you’re nearly always going to have some low p-value false positives in a screen like this if you include enough features. It’s possible to end up with a situation where absolutely nothing passes the threshold after correction numerically, but something strange is usually happening to get that in this biological contextI don't think this is correct. For example, if all the findings are actually null, which would result in a uniform distribution of p-values between 0 and 1, there's a good chance nothing will pass the FDR threshold.
I verified with some quick code which output a minimum q-value of 0.31 if the p-values aren't skewed at all to low values:
Python:In [1]: import numpy as np ...: from scipy.stats import false_discovery_control ...: ...: ps = np.random.uniform(0, 1, 10000) ...: ...: fdr_ps = false_discovery_control(ps, method='bh') ...: ...: np.min(fdr_ps) Out[1]: 0.31231890557870123
There still might be an issue somewhere, but it's theoretically possible with regard to q-value.
[Edit: and I’m probably adding to the conclusion by using my terminology loosely here]
Last edited:
jnmaciuch
Senior Member (Voting Rights)
Actually thanks @forestglip for prompting me to go back and check this—looking at some of my old datasets I do see a scenario where I got nothing past the q value threshold in a mouse study with very small n. So I take back my original comment
I think what tends to happen in human cohorts of disease with decent n is that when you measure enough things, you can virtually guarantee “real” differences in a subset of metabolites simply due to confounders that you couldn’t possibly account for with the study design. Less activity even when you use sedentary controls, higher proportion of sick people being prescribed psychiatric medications, more people with dietary restrictions in the sick group, etc.
Even in the context of pre vs post exercise within ME/CFS, there’s going to be differences just from day to day biological variation. Usually it’s a small enough confounding effect that it’ll wash out anyways with stringent p value correction, but it does mean that you can expect the raw p-values to skew low before correction if that makes sense. So that’s one reason why this data set off alarm bells. Not so much a statistical argument rather than an interpretive one
I think what tends to happen in human cohorts of disease with decent n is that when you measure enough things, you can virtually guarantee “real” differences in a subset of metabolites simply due to confounders that you couldn’t possibly account for with the study design. Less activity even when you use sedentary controls, higher proportion of sick people being prescribed psychiatric medications, more people with dietary restrictions in the sick group, etc.
Even in the context of pre vs post exercise within ME/CFS, there’s going to be differences just from day to day biological variation. Usually it’s a small enough confounding effect that it’ll wash out anyways with stringent p value correction, but it does mean that you can expect the raw p-values to skew low before correction if that makes sense. So that’s one reason why this data set off alarm bells. Not so much a statistical argument rather than an interpretive one
Yeah, I wouldn't expect there to be actually zero real difference between days because I expect exercise to do things to anyone, so would expect some skew in the raw p-values, though maybe not so much it passes correction.Even in the context of pre vs post exercise within ME/CFS, there’s going to be differences just from day to day biological variation. Usually it’s a small enough confounding effect that it’ll wash out anyways with stringent p value correction, but it does mean that you can expect the raw p-values to skew low before correction if that makes sense. So that’s one reason why this data set off alarm bells. Not so much a statistical argument rather than an interpretive one
But I agree it seems very strange to have so many very significant metabolites in one group and not a single significant metabolite in the other. I would assume, and this could be wrong, that those 255 significant metabolites in controls are parts of multiple unrelated pathways that get changed due to exercise or other confounders in various ways, so it'd be surprising for not one of these pathways to change between days in another group.
Edit: I mean, I don't know much about the physiology of exercise, but maybe this could be real? Maybe all these metabolites represent downstream consequences of one specific process that happens due to exercise in healthy people, and this is what is specifically not working in ME/CFS, or maybe is delayed? I hope we can get an attempt at replication, in one form or another.
Last edited:
jnmaciuch
Senior Member (Voting Rights)
What could be plausible is that a bunch of metabolites are induced less in ME/CFS relative to how much they are induced in healthy people (and the converse, metabolites that are downregulated less than they would be in healthy people). Which would also mean that we’d probably see some signs of abnormal metabolism somewhere—as various systems reflect insufficient adaption to increased strain.Edit: I mean, I don't know much about the physiology of exercise, but maybe this could be real? Maybe all these metabolites represent downstream consequences of one specific process that happens due to exercise in healthy people, and this is what is specifically not working in ME/CFS, or maybe is delayed? I hope we can get an attempt at replication, in one form or another.
But no, the idea of absolutely nothing changing after maximal exercise is unbelievably improbable to me when you can expect to have even a few significant differences in a screen like this just from sampling the same people at timepoints a few hours apart
jnmaciuch
Senior Member (Voting Rights)
I should note that when I had a metabolomics lecture in my first year the professor started off the class by asking how many metabolites we thought would detectably change in the blood just 30 mins after eating an apple. Don’t remember the exact number but it certainly wasn’t 0.
jnmaciuch
Senior Member (Voting Rights)
Realized belatedly that I forgot to post this with my other comments:


These plots from figures 6 and 7 pretty much confirm it--there are definitely changes after exercise in the ME/CFS group, they're just all over the place so the mean difference isn't significant, [edit: or its too variable to reach significance]. The plotted metabolites are the ones with the highest mean difference between timepoints in controls, which is why the red lines show such a consistent trend.
So the interpretation that there's a lack of adaptation [edit: or no change after] exercise in ME/CFS isn't supported by the data.


These plots from figures 6 and 7 pretty much confirm it--there are definitely changes after exercise in the ME/CFS group, they're just all over the place so the mean difference isn't significant, [edit: or its too variable to reach significance]. The plotted metabolites are the ones with the highest mean difference between timepoints in controls, which is why the red lines show such a consistent trend.
So the interpretation that there's a lack of adaptation [edit: or no change after] exercise in ME/CFS isn't supported by the data.
Last edited:
DMissa
Senior Member (Voting Rights)
To me it looks like, for the metabolites where there are appreciable differences, that the HC seem to change after exercise but the ME/CFS don't. Scanning over the gradients of the lines, that is. Having looked at this for a few minutes my primary conclusion would be "ME/CFS are not changing in response to exercise where HC do".Realized belatedly that I forgot to post this with my other comments:
View attachment 30363View attachment 30364
These plots from figures 6 and 7 pretty much confirm it--there are definitely changes after exercise in the ME/CFS group, they're just all over the place so the mean difference isn't significant, [edit: or its too variable to reach significance]. The plotted metabolites are the ones with the highest mean difference between timepoints in controls, which is why the red lines show such a consistent trend.
So the interpretation that there's a lack of adaptation [edit: or no change after] exercise in ME/CFS isn't supported by the data.
jnmaciuch
Senior Member (Voting Rights)
Do you mean individually or collectively? I see quite a few instances where the ME/CFS lines have a noticeable slope, even on par with the control lines in many cases (though often in the opposite direction). [Edit: I think it becomes necessary to look at individual slopes because “levels change in different directions, canceling each other out across the group” is a v different biological story from “no change after exertion”]To me it looks like, for the metabolites where there are appreciable differences, that the HC seem to change after exercise but the ME/CFS don't. Scanning over the gradients of the lines, that is. Having looked at this for a few minutes my primary conclusion would be "ME/CFS are not changing in response to exercise where HC do".
Last edited:
I think the claim isn't so much meant to be that there is a total lack of change, but that there is a lack of change associated with exercise.So the interpretation that there's a lack of adaptation [edit: or no change after] exercise in ME/CFS isn't supported by the data.
For instance, we could imagine some variable that has a huge amount of variability day to day in most people, like step count. If we do an intervention that should have no effect on that, like a sugar pill, there will still be large changes both up and down in different participants the next day. But they'll essentially be random changes that are non-significant when testing association with the intervention, so it might be a similar situation of a researcher interpreting that as a "lack of change in steps after placebo".
Of course, this is based on not rejecting a null hypothesis, so I think would be considered rather weak evidence in support of a lack of an association.
DMissa
Senior Member (Voting Rights)
Just looking at the slopes as a whole from group to group is what I'd meantDo you mean individually or collectively? I see quite a few instances where the ME/CFS lines have a noticeable slope, even on par with the control lines in many cases (though often in the opposite direction). [Edit: I think it becomes necessary to look at individual slopes because “levels change in different directions, canceling each other out across the group” is a v different biological story from “no change after exertion”]
ScoutB
Senior Member (Voting Rights)
I second @forestglip's comment that, given we expect this data to be noisy, "no change" only makes sense when speaking about the average. Maybe there's a better term that doesn't make it sound like each MECFS patient literally experienced no change..
Have there being other urinalyses that looked before and after an exercise challenge? Or sleep, as was mentioned earlier in this thread.given that there actually have been other urinalysis studies done in ME/CFS and no smoking gun
jnmaciuch
Senior Member (Voting Rights)
When the lack of significant metabolites after exercise is referred to as “striking” and interpreted in the abstract and text as potential evidence of a failure to adapt after exercise (and “anomalous recovery” in the title), I think it is incompatible with the data just showing much more [edit: between-timepoints variability] in ME/CFS than the control groupI think the claim isn't so much meant to be that there is a total lack of change, but that there is a lack of change associated with exercise
[Edited to correct missuse of term]
Last edited:
